- About
- Blog
- Infertility
- IVF Treatments
- New IVF Advances
- IVF Treatment in Cyprus
- IVF For Women Over 40
- IVF For Women Over 50
- IUI
- Mini IVF
- IVF + ICSI
- Cytoplasmic IVF
- Tandem IVF Cycle
- Egg Donation
- Embryo Donation
- Sperm Donation
- Gender – Sex Selection
- Gestational Surrogacy
- Same Sex Gay Surrogacy
- Surgical Sperm Retrieval
- Pre Implantation Genetic Diagnosis PGD
- Egg Freezing
- Gender Selection Using Donor Eggs
- Sickle Cell Disease Prevention
- F.A.Q.
- Travel
- About
- Blog
- Infertility
- IVF Treatments
- New IVF Advances
- IVF Treatment in Cyprus
- IVF For Women Over 40
- IVF For Women Over 50
- IUI
- Mini IVF
- IVF + ICSI
- Cytoplasmic IVF
- Tandem IVF Cycle
- Egg Donation
- Embryo Donation
- Sperm Donation
- Gender – Sex Selection
- Gestational Surrogacy
- Same Sex Gay Surrogacy
- Surgical Sperm Retrieval
- Pre Implantation Genetic Diagnosis PGD
- Egg Freezing
- Gender Selection Using Donor Eggs
- Sickle Cell Disease Prevention
- F.A.Q.
- Travel
IVF For Women Over 40
IVF for Women Over 40: Realistic, Yet Hopeful Paths Forward
Understanding the Landscape:
As women advance in age, particularly beyond 40 years of age, we observe a marked decline in fertility. This is mainly because women are born with a finite number of follicles, and both the quantity and quality decrease over time due to cumulative oxidative stress, mitochondrial dysfunction, and meiotic errors (te Velde and Pearson, 2002).
According to large registry data, live birth rates using non-donor eggs fall from around 26% at ages 38–40 to approximately 13% at 41–42, and to below 5% beyond 42 years (SART, 2023). Below diagram shows a rough estimate of an average woman’s ovarian reserves throughout her reproductive years:
Figure 1: Age and Female Ovarian Reserve
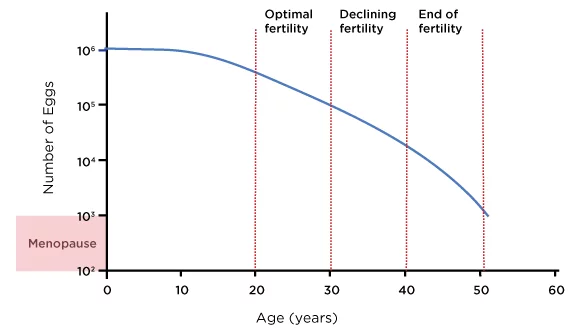
Source: Ozyigit, A. and Ozyigit, S., 2018. The IVF Guide What You Need to Know About Fertility, Infertility and Available Treatment Options. 1st ed. Irvine, CA: Universal Publishers, figure 1.
That said, ovarian reserve and response vary between individuals, and some women maintain higher reproductive potential into their early to mid forties. This makes personalised assessment via Antral Follicle Count (AFC), Anti-Müllerian Hormone (AMH) testing and prior IVF cycle outcomes essential for selecting the most appropriate strategy (Broer et al., 2014).
Oocyte or Embryo Banking: Building Your Own “Safety Net”
One practical approach to improve cumulative success rates is oocyte or embryo banking. By undergoing multiple stimulation and retrieval cycles, it is possible to accumulate a greater number of oocytes (or embryos), thereby increasing the likelihood of a successful pregnancy. Oocyte vitrification has been shown to yield survival and fertilisation rates comparable to fresh oocytes (Cobo et al., 2016) without increased risk of congenital anomalies (Noyes et al., 2009). Therefore, multiple oocyte retrievals can offer numbers that are not possibe with a sinle egg retrieval procedure.
While pregnancy and live birth rates for women aged 40+ in a single treatment cycle is relatively modest, it is important to remember that these figures are calculated per cycle. When several cycles are undertaken and results are combined, the overall likelihood of achieving a pregnancy and live birth can increase considerably. This approach also provides the opportunity to perform preimplantation genetic testing for aneuploidy (PGT-A), which can help identify embryos with a normal chromosomal complement and further improve the chances of success (Doyle et al., 2016).
Oocyte banking can be even more effective when combined with other modern advances in reproductive medicine that have the potential to enhance cycle outcomes. Among these are ovarian PRP treatment and a modern technological innovation known as Mitochondrial Replacement Therapy (MRT). Incorporating such approaches alongside oocyte banking may improve the quality and developmental potential of the eggs collected, thereby increasing the number of viable embryos available for transfer. When used strategically, these methods can complement the benefits of banking by not only expanding the pool of stored oocytes but also optimising their reproductive potential, ultimately offering a greater chance of achieving a successful pregnancy.
Ovarian Platelet-Rich Plasma (PRP)
Ovarian PRP involves injecting autologous platelet concentrate into the ovaries, aiming to stimulate local growth factors that may enhance follicular recruitment. Studies in poor responders, including women of advanced reproductive age, have reported improvements in AMH, AFC, and blastocyst quality, with some live births achieved (Sfakianoudis et al., 2019). A recent study demonstrated significant increases in usable blastocyst yield after PRP in women with prior poor-quality embryos (Yu et al., 2025). While not yet standard of care, PRP is a reasonable adjunct for selected patients wishing to optimise their own-egg potential.
Ovarian PRP therapy is a far more sophisticated procedure than simply injecting platelet-rich plasma into the ovaries and hoping for improvement. Its success depends on carefully designed protocols that begin with proper platelet preparation, including controlled inactivation to prevent premature activation and depletion of growth factors before reaching the target tissue. The concentration and density of platelets must be optimized to ensure a therapeutically meaningful dose, as suboptimal or overly dilute preparations may fail to provide the intended regenerative stimulus. Equally critical is the precision of injection with targeting specific anatomical regions within the ovary where follicular development is most likely to benefit from enhanced vascularization, cellular signaling, and microenvironmental support. Following the procedure, a tailored supplementation regimen is essential to sustain and support the oogenesis process, ensuring that the biochemical and hormonal environment is conducive to follicular recruitment, growth, and maturation. Together, these factors transform PRP therapy from a simple injection into a scientifically grounded and potentially transformative intervention for ovarian function.
Mitochondrial Replacement Therapy (MRT)
Mitochondrial Replacement Therapy (MRT) is an emerging reproductive technique aimed at restoring the bioenergetic capacity of oocytes by supplementing them with healthy donor mitochondria. This approach directly addresses one of the most significant age-related changes in female reproduction: the progressive decline in mitochondrial function, which has been strongly associated with reduced oocyte competence and impaired embryonic development (St. John et al., 2019).
By introducing functional mitochondria into the oocyte, MRT seeks to improve ATP production, optimize the cellular environment for meiotic division, and enhance the developmental potential of resulting embryos. Although its clinical application is relatively new, North Cyprus IVF Center being among the very few clinical facilities offering this service, early human and preclinical studies have demonstrated encouraging results, particularly in patients with a history of recurrent embryo developmental arrest or poor embryo quality (Zhang et al., 2017).
Dr. Shoukhrat Mitalipov and colleagues have been at the forefront of MRT research, demonstrating in both animal models and human oocytes that the transfer of healthy mitochondria can correct bioenergetic deficiencies and lead to improved fertilization and embryo development outcomes, supporting the rationale for its targeted use in cases of age or disease related mitochondrial insufficiency (Kang et al., 2016).
Combining Strategies: Oocyte Banking, Ovarian PRP Treatment and MRT
For certain patients, particularly those with a history of failed cycles due to poor embryo development, a comprehensive strategy that combines oocyte banking, ovarian PRP, and mitochondrial replacement therapy may offer the greatest potential to improve outcomes. This multi-faceted approach targets oocyte aging from several directions. Oocyte banking allows obtaining supernumerary oocytes which would not be possible with a single egg retrieval in an IVF cycle. Ovarian PRP aims to enhance the follicular environment and promote healthier follicular recruitment with an egg retrieval cycle, while mitochondrial replacement therapy works to restore optimal oocyte bioenergetics and improve embryo developmental potential. Together, these interventions address both the cellular energy production and the ovarian microenvironment, two critical determinants of reproductive success in age related infertility (Labarta et al., 2019).
****
These strategies are designed to improve the likelihood of achieving a pregnancy using a woman’s own genetic material, thereby allowing for a biological child. While such interventions can substantially increase the chances of success compared to a standard IVF cycle, they may still carry a significant financial cost and cannot guarantee a high probability of success, particularly in cases of advanced maternal age or diminished ovarian reserve. For couples or individuals who do not wish to consider donor eggs, these approaches may still provide a meaningful improvement in the odds of having a biological child. However, if the primary goal is achieving a pregnancy and welcoming a child regardless of whether there is a genetic link to the intended mother, alternative options such as a tandem IVF cycle or the direct use of donor eggs can offer substantially higher success rates, especially for women over the age of 40.
Tandem IVF Cycle
A tandem IVF cycle is a treatment approach in which a patient undergoes ovarian stimulation to produce her own eggs while simultaneously using donor eggs in the same treatment cycle. Both sets of eggs are fertilized separately, and embryos are created from each source. This allows the intended parents to attempt conception with the patient’s own genetic material while also having high-quality donor embryos available in the same cycle. Embryos from both sources can be cultured to the blastocyst stage, genetically tested if desired, and stored for future use. This approach offers the unique advantage of maximizing the chances of a successful outcome within a single treatment cycle while preserving the possibility of a biological connection to the intended mother.
Donor Egg IVF
Donor Egg IVF involves the use of oocytes from a healthy, screened donor, which are fertilized with the intended father’s sperm or donor sperm. Because egg quality is largely dependent on the age and reproductive health of the donor rather than the intended mother, this approach significantly increases the probability of producing high-quality embryos and achieving a successful pregnancy. Donor egg IVF is particularly valuable for women of advanced reproductive age, those with severely diminished ovarian reserve, or those whose previous IVF cycles with their own eggs have failed. The resulting child will share a genetic connection with the sperm source but not with the egg recipient, although the pregnancy experience and maternal–fetal bond remain fully intact.
Practical Pathways for Women Over 40
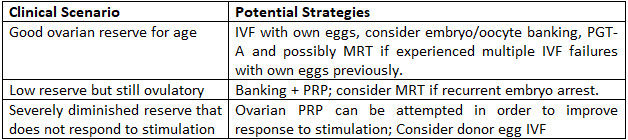
Key Takeaways
- – Success with own eggs over 40 is possible but challenging; realistic expectations are important.
- – Oocyte/embryo banking can improve cumulative chances and allow for genetic screening.
- – MRT and PRP are promising adjuncts for select cases.
- – Donor eggs offer the highest success rates and should be discussed early if prognosis is poor.
– Please contact us for more information and customized recommendations.
References
- Broer, S.L., Broekmans, F.J., Laven, J.S. and Fauser, B.C., 2014. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Human Reproduction Update, 20(5), pp.688–701.
- Cobo, A., García-Velasco, J., Coello, A., Domingo, J., Pellicer, A. and Remohí, J., 2016. Oocyte vitrification as an efficient option for elective fertility preservation. Fertility and Sterility, 105(3), pp.755–764.
- Devroey, P., Fauser, B.C. and Diedrich, K., 2011. Approaches to improve the diagnosis and management of infertility. Human Reproduction Update, 17(5), pp.455–467.
- Doyle, J.O., Richter, K.S., Lim, J., Stillman, R.J., Graham, J.R. and Tucker, M.J., 2016. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertility and Sterility, 105(2), pp.459–466.
- Labarta, E., de los Santos, M.J., Escribá, M.J., Pellicer, A. and Herraiz, S., 2019. Mitochondria as a tool for oocyte rejuvenation. Fertility and Sterility, 111(2), pp.219–226.
- Noyes, N., Porcu, E. and Borini, A., 2009. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reproductive BioMedicine Online, 18(6), pp.769–776.
- Paulson, R.J., Hatch, I.E., Lobo, R.A. and Sauer, M.V., 2002. Cumulative pregnancy success rates after oocyte donation: implications regarding endometrial receptivity. Human Reproduction, 17(5), pp.1411–1416.
- Sauer, M.V., 1990. A preliminary report on oocyte donation extending reproductive potential to women over 40. New England Journal of Medicine, 323(17), pp.1157–1160.
- Sfakianoudis, K., Simopoulou, M., Nitsos, N., et al., 2019. Autologous platelet-rich plasma treatment enables pregnancy for a woman in premature menopause. Case Reports in Women’s Health, 23, e00129.
- Sills, E.S., Rickers, N.S., Li, X. and Palermo, G.D., 2020. First data on in vitro fertilization after intraovarian injection of calcium gluconate–activated autologous platelet rich plasma. Gynecological Endocrinology, 36(6), pp.479–482.
- St. John, J.C., Facucho-Oliveira, J., Jiang, Y., Kelly, R. and Salah, R., 2019. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Human Reproduction Update, 26(1), pp.1–35.
- Yu, T.N., Wang, H.Y., Lee, T.H., et al., 2025. Intraovarian platelet-rich plasma injection significantly improves blastocyst yield in IVF patients with prior poor embryo quality. Scientific Reports, 15, 81234.
- Zhang, J., Liu, H., Luo, S., et al., 2017. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reproductive BioMedicine Online, 34(4), pp.361–368.
North Cyprus IVF Centre
North Cyprus IVF Centre is a patient focused fertility clinic, located within “Elite Research and Surgical Hospital” in Nicosia, Cyprus. Our clinic is one of the most advanced fertility clinics in the world, offering a wider selection of treatment options at affordable prices.
Contact Us
Phone
English: +90 548 875 8000
French: +90 548 876 8000
Turkish: +90 542 869 8000
Arabic: +90 548 875 8000
German: +90 548 830 1987
Russian: +90 548 828 9955
info@northcyprusivf.net
© 2020 LowCostIVF - All rights reserved. Privacy Policy


