- About
- Blog
- Infertility
- IVF Treatments
- New IVF Advances
- Mitochondrial Replacement Therapy!
- IVF Treatment in Cyprus
- IUI
- Mini IVF
- IVF + ICSI
- Cytoplasmic IVF
- Tandem IVF Cycle
- Egg Donation
- Embryo Donation
- Sperm Donation
- Gender – Sex Selection
- Gestational Surrogacy
- Same Sex Gay Surrogacy
- Surgical Sperm Retrieval
- Pre Implantation Genetic Diagnosis PGD
- Egg Freezing
- Gender Selection Using Donor Eggs
- Sickle Cell Disease Prevention
- F.A.Q.
- Travel
- About
- Blog
- Infertility
- IVF Treatments
- New IVF Advances
- Mitochondrial Replacement Therapy!
- IVF Treatment in Cyprus
- IUI
- Mini IVF
- IVF + ICSI
- Cytoplasmic IVF
- Tandem IVF Cycle
- Egg Donation
- Embryo Donation
- Sperm Donation
- Gender – Sex Selection
- Gestational Surrogacy
- Same Sex Gay Surrogacy
- Surgical Sperm Retrieval
- Pre Implantation Genetic Diagnosis PGD
- Egg Freezing
- Gender Selection Using Donor Eggs
- Sickle Cell Disease Prevention
- F.A.Q.
- Travel
Female Infertility
Diagnosis and Management
Diagnosing female infertility is often more complex than diagnosing male infertility. While male infertility can sometimes be assessed with a single semen analysis, evaluating female infertility typically requires a series of extensive tests and screenings, which may or may not yield a definitive diagnosis.
Women contribute not only the egg but also the necessary environment for an embryo to develop into a viable human being. Although female infertility is commonly linked to issues with egg health, various ovarian and uterine conditions can also impact a woman’s ability to conceive naturally. Established guidelines exist for diagnosing primary female infertility; however, any infertility evaluation should begin with a thorough medical history. The following questions can help guide the diagnosis and treatment of primary female infertility:
⦁ Have you ever been pregnant?
⦁ Have you ever had a miscarriage? If so, at what point during the pregnancy did the miscarriage occur?
⦁ Do you have a family history of infertility?
⦁ Do you have regular menstrual cycles?
⦁ Have you recently been screened for infectious diseases, including sexually transmitted diseases?
⦁ Do you own pets?
Answers to each of these questions can provide important information to the fertility specialist. For example, owning pets can increase a woman’s likelihood of acquiring infections that can cause infertility, such as toxoplasmosis. Similarly, a family history of infertility could point to genetic causes of infertility. During any infertility workup, patients should settle for nothing less than a comprehensive medical history. A copy of our medical history form is on our “Contact” page. While the questionnaire is just the beginning of an infertility workup, these questions can help identify potential causes of infertility, but the next step is to assess the reproductive physiology of both the male and female patient. In particular, hormone testing can provide information about a woman’s ovarian function and help guide treatment.
What are the common causes of female infertility?
1- Ovulation Problems
Ovulation is a critical part of the female reproductive cycle and is essential for conception. During ovulation, a mature egg is released from one of the ovaries and becomes available for fertilization by sperm. For most women, this process occurs once per cycle, typically about two weeks before the start of their menstrual period. The egg travels through the fallopian tube, where it may be fertilized, and then moves to the uterus for implantation.
When ovulation does not occur regularly or at all, it is known as an ovulatory dysfunction. Ovulatory dysfunction is one of the leading causes of female infertility, accounting for about 25-30% of infertility cases. Women experiencing ovulation problems may have irregular menstrual cycles, missed periods, or even a complete absence of menstruation (amenorrhea). However, some women with ovulatory dysfunction may still have regular menstrual cycles, making it more challenging to identify the underlying issue without further testing.
Types of Ovulation Disorders
Ovulation problems can manifest in various forms, each with different underlying causes. The most common ovulatory disorders include:
1. Anovulation: This is the complete absence of ovulation, where the ovaries do not release an egg during the menstrual cycle. Anovulation is commonly associated with hormonal imbalances, polycystic ovary syndrome (PCOS), or primary ovarian insufficiency (POI).
2. Oligo-ovulation: In this case, ovulation occurs irregularly or infrequently. Women with oligo-ovulation may have unpredictable cycles or long gaps between periods, which can make it difficult to time intercourse for conception. Oligo-ovulation is also often related to conditions like PCOS or other hormonal issues.
3. Luteal Phase Defect (LPD): This condition occurs when the luteal phase—the period after ovulation—does not produce adequate levels of progesterone to support a fertilized egg. Low progesterone levels can prevent the uterine lining from thickening sufficiently, making it difficult for an embryo to implant and develop, which can lead to early pregnancy loss.
Causes of Ovulation Problems
Ovulation problems are primarily rooted in hormonal imbalances and disruptions to the signals between the brain and reproductive organs. The most common causes of ovulation disorders include:
1. Polycystic Ovary Syndrome (PCOS): PCOS is one of the most prevalent causes of ovulatory dysfunction, affecting around 5-10% of women of reproductive age. Women with PCOS typically have elevated levels of androgens (male hormones), which interfere with ovulation. PCOS can lead to anovulation, irregular periods, and other metabolic issues, such as insulin resistance.
2. Hypothalamic Dysfunction: The hypothalamus in the brain regulates many body functions, including the release of gonadotropin-releasing hormone (GnRH), which signals the pituitary gland to produce the hormones necessary for ovulation: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Stress, extreme weight loss, excessive exercise, or eating disorders can disrupt the hypothalamus’s function, leading to irregular or absent ovulation.
3. Primary Ovarian Insufficiency (POI): Also known as premature ovarian failure, POI occurs when the ovaries lose normal function before the age of 40. This condition is sometimes due to genetic factors, autoimmune disorders, or certain medical treatments (e.g., chemotherapy). POI can lead to reduced estrogen production, irregular cycles, and, eventually, cessation of ovulation.
4. Hyperprolactinemia: Elevated levels of prolactin, a hormone responsible for milk production, can suppress ovulation by disrupting the balance of reproductive hormones. This condition can be caused by medications, pituitary tumors, or thyroid issues.
5. Thyroid Disorders: Both hyperthyroidism (overactive thyroid) and hypothyroidism (underactive thyroid) can interfere with normal menstrual and ovulatory cycles. Thyroid hormones play a crucial role in regulating the body’s reproductive system, and any imbalance can lead to ovulation issues.
Symptoms of Ovulation Problems
Symptoms of ovulation problems can vary widely depending on the specific disorder. Common symptoms that may indicate ovulatory dysfunction include:
• Irregular menstrual cycles (too long, too short, or unpredictable)
• Absence of menstruation (amenorrhea)
• Heavy or very light menstrual bleeding
• Lack of premenstrual symptoms (breast tenderness, bloating)
• Difficulty predicting ovulation (irregular basal body temperature patterns)
• Acne, excess facial or body hair, and weight gain (particularly in women with PCOS)
These symptoms do not confirm an ovulation problem but can be indicators that warrant further evaluation by a healthcare professional.
Diagnosing Ovulation Problems
To diagnose ovulation disorders, a series of evaluations and tests can be performed. These include:
1. Medical History and Physical Examination: A thorough medical history and physical exam can reveal potential underlying causes, such as lifestyle factors, weight changes, or stress levels that might influence ovulation.
2. Ovulation Tracking: Women may be asked to track their menstrual cycles, basal body temperature, or use ovulation predictor kits. Progesterone blood tests taken during the luteal phase can confirm whether ovulation has occurred.
3. Hormonal Testing: Blood tests can evaluate levels of key hormones, including FSH, LH, estrogen, prolactin, testosterone, and thyroid hormones, to determine any imbalances that could be impacting ovulation.
4. Ultrasound: A transvaginal ultrasound can help visualize the ovaries and detect the presence of follicles, cysts, or other abnormalities. This imaging can be particularly useful in diagnosing PCOS or monitoring the ovaries’ response to hormonal treatment if fertility treatment is being performed.
5. Additional Tests for Underlying Conditions: If initial testing points to an issue like POI, PCOS, or a thyroid disorder, additional tests may be conducted to confirm the diagnosis.
Ovulation problems can make conception challenging, as irregular or absent ovulation reduces the number of opportunities for an egg to be fertilized. Even when ovulation occurs, conditions like Luteal Phase Defect or low-quality eggs may interfere with implantation or increase the risk of miscarriage. Fortunately, many ovulation disorders are treatable, and advances in fertility medicine offer various options for women experiencing ovulation-related infertility.
2- Problems with Fallopian Tubes
Blocked or damaged fallopian tubes are a significant cause of female infertility, as they disrupt the normal pathway for the egg and sperm to meet, thereby preventing fertilization. The fallopian tubes, which connect the ovaries to the uterus, play a crucial role in the reproductive process. Each month, after ovulation, an egg is released from one of the ovaries and travels through the fallopian tube, where it may encounter sperm and undergo fertilization. Once fertilized, the embryo continues to move through the tube until it reaches the uterus for implantation. When one or both fallopian tubes are blocked or damaged, this journey is impeded, making natural conception difficult or impossible.
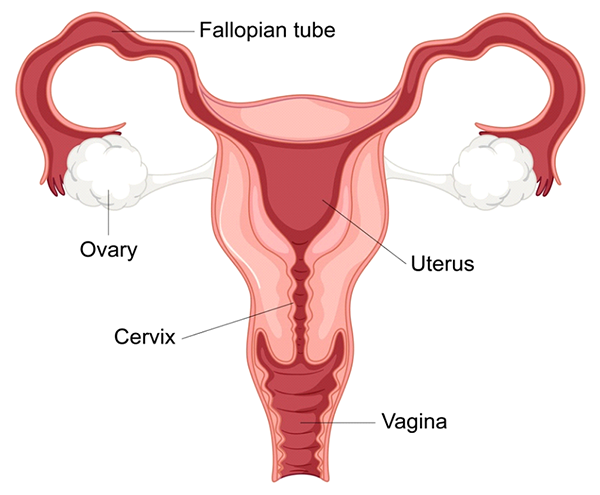
How Fallopian Tube Damage Affects Fertility
Fallopian tube damage or blockages can affect fertility by:
a) Preventing Sperm from Reaching the Egg: If the fallopian tubes are fully blocked, sperm cannot reach the egg, and fertilization cannot occur. This situation is known as tubal factor infertility, which accounts for about 25-35% of female infertility cases.
b) Interfering with Egg Pickup: Damage to the fimbriae (the fringed ends of the fallopian tubes near the ovaries) can prevent the egg from being captured and drawn into the fallopian tube after ovulation. Without this process, the egg cannot travel down the tube to meet the sperm.
c) Preventing Embryo Transport: If a partially blocked tube allows fertilization but impedes the passage of the fertilized egg (embryo), it may result in an ectopic pregnancy. This is a serious condition where the embryo implants and grows within the fallopian tube rather than the uterus, which can lead to medical complications and requires prompt treatment.
d) Inhibiting Implantation in the Uterus: Even if the tube is only partially blocked or damaged, it may affect the embryo’s timely transport to the uterus. Delayed embryo transport may reduce the likelihood of successful implantation or increase the risk of implantation in an inappropriate location.
Causes of Blocked or Damaged Fallopian Tubes
1. Pelvic Inflammatory Disease (PID): PID is an infection of the female reproductive organs, often caused by sexually transmitted infections (STIs) like chlamydia or gonorrhea. Left untreated, these infections can lead to inflammation and scarring of the fallopian tubes, causing partial or complete blockages. PID is one of the most common causes of tubal factor infertility.
2. Endometriosis: Endometriosis is a condition where tissue similar to the lining of the uterus grows outside the uterus, often on the ovaries, fallopian tubes, or pelvic lining. Endometrial implants on or near the fallopian tubes can cause adhesions, inflammation, or scarring, leading to blockages or restricted movement of the tubes.
3. Tubal Surgery: Previous surgeries on the fallopian tubes, such as procedures to repair a tubal pregnancy, remove cysts, or treat tubal infections, can lead to scarring and adhesions that block the tubes. Women who have undergone tubal ligation (surgical sterilization) may also experience infertility due to intentional blockage of the tubes.
4. Adhesions from Abdominal or Pelvic Surgery: Surgeries involving the abdomen or pelvis, such as appendectomy, cesarean section, or surgeries for ovarian cysts, can cause scar tissue to form around the fallopian tubes. These adhesions may obstruct the tubes or restrict their movement, affecting the egg’s journey to the uterus.
5. Hydrosalpinx: Hydrosalpinx is a condition where one or both fallopian tubes become blocked and filled with fluid. This condition is often a result of infections, PID, or endometriosis. The fluid within a hydrosalpinx can be toxic to embryos and may reduce the success rate of in vitro fertilization (IVF) if left untreated.
6. Congenital Abnormalities: In rare cases, some women may be born with structural abnormalities in their fallopian tubes, which can lead to blockages or dysfunction.
Diagnosing Blocked or Damaged Fallopian Tubes
To diagnose blockages or damage in the fallopian tubes, the following diagnostic methods are often used:
1. Hysterosalpingography (HSG): HSG is an X-ray procedure in which a special dye is injected into the uterus and fallopian tubes to evaluate their structure. The dye shows up on X-rays and can reveal blockages or abnormalities in the tubes.
2. Sonohysterography: This procedure is similar to HSG but uses ultrasound instead of X-rays. A saline solution is injected into the uterus, allowing the healthcare provider to view the fallopian tubes and assess for any blockages or damage.
3. Laparoscopy: Laparoscopy is a minimally invasive surgical procedure that involves inserting a small camera through a tiny incision in the abdomen. This procedure allows the healthcare provider to view the fallopian tubes directly, remove minor blockages or adhesions, and evaluate the condition of surrounding pelvic organs. Laparoscopy is often performed when other diagnostic tests have been inconclusive or when symptoms of endometriosis or pelvic adhesions are present.
4. Chlamydia Antibody Test: Since chlamydia infection can cause tubal damage without obvious symptoms, this blood test checks for antibodies to chlamydia. A positive result may indicate a past infection that could have affected the tubes.
IVF is often recommended for women with blocked or severely damaged fallopian tubes, as it bypasses the need for tubes entirely. In IVF, eggs are retrieved from the ovaries, fertilized with sperm in a lab, and the resulting embryos are transferred directly to the uterus. IVF is considered the most effective treatment for tubal factor infertility, particularly in cases of severe or irreversible damage. Women with one blocked tube may still try to conceive naturally, but IVF provides an alternative if other efforts are unsuccessful.
3- Physical Problems with the Uterus
Physical abnormalities or structural problems in the uterus are another significant factor in female infertility. The uterus, or womb, provides the environment for embryo implantation and the growth of a developing fetus. Structural issues with the uterus can disrupt the implantation of a fertilized egg, affect embryo development, or increase the risk of miscarriage. These issues are often referred to as uterine factor infertility, and they can impact both the ability to conceive and the likelihood of maintaining a healthy pregnancy.
Types of Uterine Structural Problems and Their Impact on Fertility
There are several types of physical problems that can affect the uterus and lead to infertility. Each condition has unique effects on fertility, depending on its location, severity, and underlying cause. The most common uterine structural problems associated with infertility include:
1. Fibroids (Uterine Leiomyomas):
Fibroids are noncancerous growths made of muscle and fibrous tissue that develop in or on the walls of the uterus. While fibroids are quite common, affecting up to 70-80% of women by age 50, they don’t always cause infertility. The impact of fibroids on fertility depends on their size, number, and location within the uterus. Submucosal fibroids, which grow within the uterine cavity, are more likely to interfere with implantation or embryo development. Large fibroids or multiple fibroids may also alter the shape of the uterine cavity or block the fallopian tubes, further hindering conception. Fibroids can also increase the risk of miscarriage, preterm birth, and complications during pregnancy, depending on their size and location.
2. Uterine Polyps:
Uterine polyps are small, benign growths that arise from the lining of the uterus (endometrium). Although generally not cancerous, polyps can interfere with fertility by blocking the passage of sperm, preventing implantation, or disrupting the blood supply to an implanted embryo. Polyps can be especially problematic if they are located in the proximity of where an embryo would typically implant. In such cases, they may prevent a successful pregnancy or increase the risk of early miscarriage.
3. Congenital Uterine Anomalies:
These are structural abnormalities in the uterus present from birth, caused by developmental issues in the formation of the Müllerian ducts, which give rise to the uterus, fallopian tubes, and upper portion of the vagina. Congenital uterine anomalies can take one of the following several forms:
Bicornuate Uterus: The uterus has a heart-like shape with a deep indentation at the top, which creates two cavities. A bicornuate uterus can increase the risk of miscarriage, preterm birth, and abnormal fetal positioning.
Septate Uterus: A fibrous or muscular septum (wall) divides the uterine cavity, partially or fully. This condition is associated with higher rates of miscarriage and implantation failure, as the septum may have a reduced blood supply, making it less conducive for embryo implantation.
Unicornuate Uterus: Only half of the uterus is formed, often resulting in a smaller than usual uterine cavity. This can lead to difficulties in sustaining a pregnancy, with a higher risk of miscarriage and preterm delivery.
Didelphys Uterus: This rare condition, also known as a “double uterus,” occurs when two separate uterine cavities form. Although conception is possible, pregnancy may be higher-risk due to limited uterine space.
Congenital uterine anomalies often contribute to recurrent miscarriages and may also make it more difficult for women to conceive, especially if the uterine shape prevents proper implantation.
4. Intrauterine Adhesions (Asherman Syndrome):
Intrauterine adhesions are bands of scar tissue that form within the uterus, often as a result of trauma to the uterine lining. They are commonly caused by uterine surgeries, such as dilation and curettage (D&C), or infections. Asherman syndrome can lead to partial or total obstruction of the uterine cavity, which can prevent implantation or lead to recurrent miscarriage. In severe cases, the adhesions may prevent menstruation entirely (amenorrhea). Women with Asherman syndrome may experience light or absent periods, pelvic pain, and infertility, especially if the adhesions disrupt normal uterine function.
5. Adenomyosis:
Adenomyosis occurs when endometrial tissue (the lining of the uterus) grows into the muscular wall of the uterus. This condition can cause a thickened, enlarged uterus and lead to painful or heavy periods, pelvic pain, and infertility. Adenomyosis is usually associated with implantation failures and an increased risk of miscarriage. While the exact cause of infertility in adenomyosis is not fully understood, it is believed that the abnormal growth of endometrial tissue in the muscle wall disrupts proper functioning of the uterus.
6. Endometrial Issues:
The thickness and quality of the endometrial lining are crucial for successful embryo implantation. In some cases, women may have an endometrial lining that is too thin or fails to develop adequately, often due to hormonal imbalances, certain medications, or previous uterine surgeries. A thin endometrium may reduce the likelihood of implantation, as it may not provide the necessary support for an embryo to attach and thrive.
Symptoms of Uterine Structural Problems
While some women with uterine structural problems may have no symptoms, others may experience the following:
• Irregular or heavy menstrual bleeding (common with fibroids, polyps, and adenomyosis)
• Painful periods or pelvic pain
• Recurrent pregnancy loss or miscarriage
• Difficulty conceiving despite regular ovulation and sperm health
• Pain during sexual intercourse
Because symptoms can be subtle or overlap with other conditions, uterine structural problems are often only diagnosed after a woman seeks evaluation for infertility or recurrent miscarriages.
Diagnosing Uterine Structural Problems
To diagnose structural issues in the uterus, a combination of imaging tests and, if needed, minimally invasive procedures are often used.
Ultrasound: Transvaginal ultrasound is a first-line imaging tool for assessing uterine structure. It can identify fibroids, polyps, congenital abnormalities, and adenomyosis. In some cases, 3D ultrasound may provide a more detailed view of the uterine shape.
Hysterosalpingography (HSG): This X-ray procedure involves injecting a contrast dye into the uterus to outline the shape of the uterine cavity and check for blockages or adhesions in the fallopian tubes.
Sonohysterography: A saline solution is injected into the uterus during an ultrasound to give a clearer view of the uterine cavity. This technique can help identify polyps, fibroids, and adhesions.
Magnetic Resonance Imaging (MRI): MRI provides high-resolution images of the uterus and is particularly useful for diagnosing adenomyosis, congenital anomalies, and deep uterine fibroids.
Hysteroscopy: Hysteroscopy involves inserting a thin, lighted scope through the cervix to view the inside of the uterus directly. It allows the provider to visually inspect the uterine cavity for polyps, fibroids, or adhesions and, if necessary, remove or treat them during the same procedure.
Laparoscopy: Laparoscopy is a minimally invasive surgical procedure that allows the provider to view the outside of the uterus and surrounding structures, such as the fallopian tubes and ovaries. It is particularly helpful for identifying endometriosis or pelvic adhesions that may affect fertility.
4- Problems with the Cervix
Cervical problems can significantly impact a woman’s fertility, as the cervix plays a critical role in the journey of sperm to the egg. The cervix is the lower part of the uterus that connects to the vagina, and it serves as the gateway for sperm to enter the uterus and move up toward the fallopian tubes, where fertilization typically occurs. Cervical issues that interfere with the function or structure of the cervix can prevent sperm from reaching the egg, reduce the likelihood of implantation, or increase the risk of pregnancy loss.

Types of Cervical Problems and Their Impact on Fertility
There are several types of cervical issues that can affect fertility:
1. Cervical Mucus Abnormalities:
Cervical mucus, produced by glands within the cervix, is crucial for facilitating sperm movement. Around the time of ovulation, cervical mucus becomes thin, stretchy, and slippery (similar to the consistency of egg whites), making it easier for sperm to travel through the cervix and into the uterus. When cervical mucus is too thick, scant, or hostile (unfavorable to sperm), it can hinder sperm movement or kill sperm before they reach the egg. This condition is sometimes called “hostile cervical mucus” and is often due to hormonal imbalances, infections, certain medications, or underlying health conditions. Insufficient or poor-quality cervical mucus may also prevent sperm from surviving long enough to reach the egg, reducing the likelihood of fertilization.
2. Cervical Stenosis (Narrowing of the Cervical Canal):
Cervical stenosis is a condition where the cervical canal becomes abnormally narrow or closed, making it difficult for sperm to pass through. This condition can be congenital (present from birth) or acquired due to surgeries, infections, trauma, or radiation therapy. In severe cases, cervical stenosis can completely obstruct the passage of sperm, effectively preventing natural conception. Cervical stenosis may also lead to menstrual irregularities and pain, as menstrual blood can have difficulty passing through the narrowed cervix. In cases of severe stenosis, assisted reproductive techniques like intrauterine insemination (IUI) or in vitro fertilization (IVF) may be recommended to bypass the cervix.
3. Cervical Infections and Inflammation:
Infections of the cervix, such as cervicitis (inflammation of the cervix) or sexually transmitted infections (STIs) like chlamydia and gonorrhea, can impact fertility. Inflammation and infection in the cervix can alter the quality of cervical mucus, making it less favorable for sperm. In some cases, untreated infections can lead to the development of scar tissue, which may narrow the cervical canal. Infections can also spread upward, leading to pelvic inflammatory disease (PID), which can further impact fertility by causing scarring and blockages in the fallopian tubes.
4. Cervical Insufficiency (Incompetent Cervix):
Cervical insufficiency, also known as an incompetent cervix, occurs when the cervix is too weak to remain closed during pregnancy. Although this condition doesn’t directly prevent conception, it can lead to miscarriage, especially in the second trimester, as the cervix may start to dilate prematurely. Cervical insufficiency may be due to genetic factors, previous cervical surgeries (e.g., LEEP procedure or cone biopsy), trauma from childbirth, or congenital abnormalities. Women with this condition often experience recurrent pregnancy loss and may require medical interventions to support the cervix during pregnancy.
5. Previous Cervical Surgeries or Procedures:
Certain cervical procedures, such as a LEEP (loop electrosurgical excision procedure) or cone biopsy, are used to remove precancerous cells from the cervix but may sometimes affect fertility. These procedures can weaken the cervix or cause scarring, which may lead to cervical insufficiency or stenosis. While most women retain fertility after these procedures, those who experience complications may face a higher risk of infertility, recurrent pregnancy loss, or preterm birth. In some cases, cervical scarring from surgery can impact mucus production or make it difficult for sperm to pass through.
6. Antisperm Antibodies in Cervical Mucus:
In rare cases, a woman’s immune system may produce antibodies against sperm, perceiving them as foreign invaders. These antibodies can be present in the cervical mucus and can impair sperm movement, damage sperm, or prevent sperm from reaching the uterus. The presence of antisperm antibodies in cervical mucus can lead to what’s known as “immunological infertility,” which may require specific treatments, such as IUI or IVF, to help bypass the cervix and improve the chances of fertilization.
Diagnosing Cervical Problems in Infertility
Diagnosing cervical-related infertility typically involves a series of tests and evaluations. A pelvic exam allows the healthcare provider to assess the cervix’s size, shape, and position, and check for any visible abnormalities or signs of inflammation. Sometimes, a postcoital test can be ordered if hostile mucus is suspected. In this test, a sample of cervical mucus is collected after intercourse to examine sperm movement within the mucus. This test helps assess if cervical mucus is supporting or inhibiting sperm movement, though it’s less commonly used today due to variable results.
HSG is an X-ray procedure that uses contrast dye to evaluate the shape of the uterus and the patency (openness) of the cervical canal and fallopian tubes. It helps detect any structural blockages in the cervix. Cervical cultures may be performed to identify infections, while a cervical biopsy can evaluate the presence of any abnormal or precancerous cells. These tests can help identify infections or scarring that may be impacting fertility.
Ultrasound imaging may be used to evaluate the cervix’s structure and length, especially in cases where cervical insufficiency is suspected. Transvaginal ultrasound is often used to monitor cervical length during pregnancy in women with a history of cervical insufficiency.
5- Age
Age is one of the most significant factors affecting female fertility. As a woman ages, her fertility naturally declines due to changes in both the quantity and quality of her eggs, as well as alterations in her reproductive system that impact conception and pregnancy. The decline in fertility typically begins in a woman’s late 20s to early 30s and accelerates significantly after the age of 35. By the time a woman reaches her 40s, the likelihood of conceiving naturally drops considerably, and the risk of complications increases. The following changes occur as a woman’s age increases:
1. Decline in Egg Quantity (Ovarian Reserve)
Women are born with all the eggs they will ever have, approximately 1-2 million at the time of birth. By puberty, this number has already decreased to around 300,000 to 400,000, and with each menstrual cycle, more eggs are depleted. By the late 30s, the number of remaining eggs (known as the ovarian reserve) declines rapidly. As a woman approaches menopause, typically around the early 50s, very few, if any, viable eggs remain. The decrease in ovarian reserve affects a woman’s ability to conceive, as fewer eggs mean fewer opportunities for fertilization. Reduced egg supply also limits the effectiveness of fertility treatments like in vitro fertilization (IVF) because the ovaries may not produce enough eggs in response to stimulation.
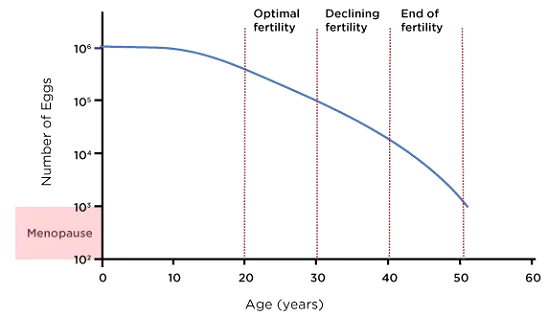
2. Decline in Egg Quality
Not only does the quantity of eggs decrease with age, but so does their quality. Egg quality refers to the genetic and chromosomal integrity of an egg. As women age, eggs are more likely to accumulate chromosomal abnormalities, increasing the likelihood of genetic disorders, such as Down syndrome, and reducing the chances of successful conception. Chromosomal abnormalities also increase the risk of miscarriage, particularly in women over the age of 35. Studies show that the miscarriage rate for women in their early 20s is about 10-15%, but this rate rises to around 35% for women in their early 40s.
Not only genetic problems are prevalent in the oocyte in older age brackets, but the cytoplasmic organelles also lose their function with increasing age. Especially mitochondrial function is vital for cellular growth and development and aging mitochondria can fail to provide the necessary balance between energy production and oxidative damage inside the oocytes. Some new generation treatments such as “Mitochondrial Replacement Therapy” can be helpful in cases where oocyte aging is a concern during fertility treatments. The decline in egg quality is a significant reason why pregnancy and live birth rates drop with age, even with the use of fertility treatments.
3. Increased Risk of Reproductive Health Issues
As women age, they are more likely to develop health conditions that can impact fertility, including endometriosis, uterine fibroids and chronic complications of pelvic inflammatory disease. These health issues not only affect the chances of conception but can also increase the risk of pregnancy complications and miscarriage.
4. Reduced Uterine Receptivity
With age, the uterus may also experience changes that impact its ability to support a pregnancy. The endometrial lining, which thickens each cycle to prepare for a potential embryo, may not develop as optimally as it once did. This change can reduce the likelihood of successful implantation, even if a healthy embryo is present. The vascular system in the uterus may also be affected by age, reducing blood flow to the endometrial lining. Adequate blood flow is essential for a receptive uterus that can support an embryo, so diminished blood flow can reduce fertility and increase the risk of early pregnancy loss.
5. Higher Risk of Pregnancy Complications
Older women who conceive naturally or with assistance face a higher risk of pregnancy complications. These complications include:
Gestational Diabetes: Women over 35 are at increased risk of developing gestational diabetes, which can impact both maternal and fetal health.
Hypertension and Preeclampsia: High blood pressure and preeclampsia (a potentially dangerous pregnancy complication characterized by high blood pressure) are more common in older mothers.
Placenta Previa: Women over 35 have an increased risk of placenta previa, a condition where the placenta covers the cervix, which can lead to complications during delivery.
Preterm Birth: Women over 35 are at a higher risk of delivering prematurely, which can impact the health of the baby.
These pregnancy complications, while not directly impacting the ability to conceive, underscore the additional health risks faced by women attempting to conceive at an older age. Age affects not only natural conception but also the success rates of assisted reproductive technologies like IVF. Women under 35 generally have higher success rates with IVF compared to women over 40. By age 43, the success rate of IVF with a woman’s own eggs drops significantly, largely due to diminished egg quantity and quality. To increase their chances, some older women opt to use donor eggs from a younger woman, which greatly improves success rates because donor eggs are typically of higher quality. However, the process of egg donation is expensive and requires careful consideration and preparation.
Fertility Preservation Options: Egg Freezing and Embryo Freezing
With the decline in fertility associated with age, some women opt to preserve their fertility through egg or embryo freezing (cryopreservation) when they are younger, especially if they plan to delay pregnancy.
Egg Freezing: This process involves stimulating the ovaries to produce multiple eggs, which are then retrieved and frozen for future use. Egg freezing can allow women to use younger, healthier eggs when they are ready to conceive, increasing their chances of a successful pregnancy later in life.
Embryo Freezing: Similar to egg freezing, this process involves fertilizing the retrieved eggs with sperm before freezing. This option is often chosen by women who are in a committed relationship and want to have embryos ready for implantation in the future.
These techniques provide options for women who wish to delay pregnancy while preserving the quality of their eggs or embryos. While age is a major factor in fertility, medical advancements such as ART, egg freezing, and embryo freezing provide options to help women conceive later in life. Understanding the effects of age on fertility can help women make informed reproductive choices and seek assistance early if they plan to conceive at an older age.
6- Immune System Problems
Immune issues can significantly impact fertility in women, often leading to unexplained infertility, recurrent pregnancy loss, or complications in pregnancy. The immune system is designed to protect the body from infections and foreign invaders, but in some cases, immune responses can interfere with fertility by targeting reproductive cells, tissues, or even the embryo. These immune-related fertility issues can result from autoimmune diseases, immune responses to sperm or embryos, or abnormal immune system activation.
1. Autoimmune Disorders and Infertility
In autoimmune disorders, the immune system mistakenly attacks the body’s own tissues as if they were foreign invaders. Several autoimmune conditions are associated with infertility:
Systemic Lupus Erythematosus (SLE): Lupus is an autoimmune disease that can cause inflammation throughout the body, including in the reproductive organs. Women with lupus may experience menstrual irregularities, recurrent miscarriage, or preterm birth due to immune system attacks on the uterus or placenta.
Antiphospholipid Syndrome (APS): APS is an autoimmune disorder where the immune system produces antibodies against phospholipids, molecules in cell membranes, and it’s often associated with recurrent pregnancy loss. These antiphospholipid antibodies can lead to blood clot formation in the placenta, cutting off blood supply to the fetus and causing miscarriage. APS is also linked to other complications, such as preeclampsia and preterm birth.
Hashimoto’s Thyroiditis and Graves’ Disease: Both are autoimmune thyroid disorders where the immune system attacks the thyroid gland, either reducing (Hashimoto’s) or increasing (Graves’) thyroid hormone production. Thyroid hormones play a critical role in regulating the menstrual cycle and supporting pregnancy. Women with thyroid disorders often experience menstrual irregularities, ovulation issues, and a higher risk of miscarriage.
Rheumatoid Arthritis (RA) and Other Autoimmune Diseases: RA and similar autoimmune diseases can create inflammatory conditions that interfere with ovulation, embryo implantation, and pregnancy maintenance. Additionally, some medications used to treat autoimmune diseases may negatively affect fertility.
2. Anti-Sperm Antibodies
In some cases, a woman’s immune system can produce antibodies that specifically target sperm. This response may happen if sperm are exposed to the immune system through trauma, infection, or surgery. The antibodies can impair sperm motility (making it difficult for sperm to swim), bind to the sperm’s surface, or even destroy the sperm altogether, thus preventing fertilization. Anti-sperm antibodies are usually found in the cervical mucus, but they can also be present in other areas of the reproductive system. Women with anti-sperm antibodies may require treatments like intrauterine insemination (IUI) or in vitro fertilization (IVF) to bypass the cervical mucus and improve the chances of conception.
3. Anti-Ovarian Antibodies
Anti-ovarian antibodies are antibodies that attack the ovarian tissue, targeting components like egg cells, follicle-stimulating hormone (FSH) receptors, or the structures that support egg development. This immune response can interfere with ovarian function, reduce egg quality, and lead to early ovarian aging or failure (known as primary ovarian insufficiency or POI). Women with anti-ovarian antibodies may experience irregular menstrual cycles, anovulation (absence of ovulation), or diminished ovarian reserve, all of which can reduce fertility and make conception more challenging. Autoimmune ovarian failure is sometimes linked to other autoimmune conditions, such as Addison’s disease, lupus, or thyroid disorders.
4. Endometriosis and Inflammation
Endometriosis is a condition in which tissue similar to the uterine lining (endometrium) grows outside the uterus, often on the ovaries, fallopian tubes, and other pelvic organs. While endometriosis is not strictly an autoimmune disease, it has been found to involve immune system dysfunction and chronic inflammation. In women with endometriosis, the immune system fails to clear the misplaced endometrial tissue, leading to persistent inflammation. This inflammatory environment can interfere with egg quality, disrupt ovulation, and create adhesions (scar tissue) that block or damage the fallopian tubes. The inflammation and immune activity associated with endometriosis can also create a hostile environment for sperm, eggs, and embryos, making it harder to achieve a successful pregnancy.
5. Natural Killer (NK) Cells and Implantation Failure
Natural killer (NK) cells are immune cells that play an essential role in the body’s defense mechanisms, including regulating immune responses in the uterus. In a typical pregnancy, uterine NK cells help support implantation by promoting blood flow to the developing embryo. However, some women may have elevated levels of NK cells or abnormally activated NK cells that target and attack the embryo, leading to implantation failure or early pregnancy loss. Elevated NK cell activity is associated with recurrent miscarriage and may contribute to unexplained infertility. Testing for NK cell activity is controversial, as not all researchers agree on its impact on fertility, but some fertility specialists may offer immune-modulating treatments, such as steroids or intravenous immunoglobulin (IVIG), to reduce NK cell activity and improve implantation success.
6. Cytokine Imbalance and Inflammatory Response
Cytokines are small proteins produced by immune cells that play a role in cell signaling and immune regulation. During early pregnancy, a balanced cytokine environment is necessary to support embryo implantation and early fetal development. However, an imbalance in cytokines—especially an increase in pro-inflammatory cytokines—can disrupt this process. In women with immune-related infertility, elevated levels of inflammatory cytokines may create an unfavorable uterine environment, reducing the chances of embryo implantation or increasing the risk of miscarriage. Conditions like autoimmune diseases, infections, or chronic inflammation may lead to a cytokine imbalance, which can contribute to infertility or recurrent pregnancy loss.
7. Immune Response to the Placenta
The placenta, which nourishes the developing fetus, is crucial for a successful pregnancy. During normal pregnancy, the maternal immune system tolerates the placenta, which has genetic material from both the mother and the father. However, in some women, the immune system may view the placenta as foreign and initiate an immune response against it. This immune response can restrict blood flow to the placenta, increasing the risk of miscarriage, preeclampsia, intrauterine growth restriction, and other pregnancy complications. Conditions like APS (antiphospholipid syndrome) are classic examples where immune responses can interfere with placental function, leading to pregnancy loss.
8. Immunological Implantation Dysfunction
Some women with unexplained infertility may have what’s termed immunological implantation dysfunction. This term refers to an overactive or inappropriate immune response that prevents an embryo from successfully implanting in the uterus, even if both the embryo and the uterine lining are healthy. Immunological implantation dysfunction is still an area of research, but it is believed to involve abnormal activity of immune cells, including NK cells, T cells, and macrophages, or an imbalance in immune-modulating factors within the uterus. Treatment options are still being developed, but some fertility specialists may use corticosteroids, IVIG, or other immune-modulating therapies to attempt to improve implantation success.
Diagnostic Testing for Immune-Related Infertility
Testing for immune-related infertility can involve several approaches, depending on the suspected immune issue:
Blood Tests: Blood tests can detect specific antibodies (e.g., anti-sperm antibodies, anti-ovarian antibodies, antiphospholipid antibodies) and measure immune cell levels or activity.
NK Cell Assays: These tests evaluate natural killer cell levels and activity in the blood or uterus, though their clinical utility is still debated.
Cytokine Profile Testing: This test measures levels of pro-inflammatory and anti-inflammatory cytokines to assess immune balance, particularly in women with recurrent pregnancy loss.
Hysteroscopy or Biopsy: A hysteroscopy (viewing the uterus with a camera) or endometrial biopsy may be performed to evaluate inflammation or immune activity directly in the uterus.
Treatment Options for Immune-Related Infertility
Treatments for immune-related infertility aim to address the specific immune issue and improve the chances of a successful pregnancy:
Immunomodulatory Medications:
• Corticosteroids: Low-dose steroids like prednisone may be used to reduce immune activity and inflammation, particularly in cases of NK cell elevation or immune-related implantation failure.
• Intravenous Immunoglobulin (IVIG): IVIG is used in some cases to suppress abnormal immune responses, especially in women with recurrent miscarriage or NK cell elevation, though its use is still experimental.
• Low-Molecular-Weight Heparin (LMWH) and Aspirin: In cases of APS or clotting disorders, blood-thinning medications like heparin and aspirin can reduce the risk of blood clots, supporting placental health and reducing miscarriage risk.
Antibiotics for Infections: In cases of cervicitis or low-grade infections, antibiotics can reduce inflammation in the reproductive system, potentially improving fertility.
Assisted Reproductive Techniques (ART):
In cases where immune issues are impacting natural conception, ART options like IVF may bypass some immune barriers by allowing fertilization to occur outside the body. Techniques like intracytoplasmic sperm injection (ICSI) may be used in cases of anti-sperm antibodies, and embryo transfer can bypass hostile cervical mucus or abnormal immune responses at the cervix.
7- Thrombophilia Defects
Thrombophilia, a condition characterized by an increased tendency for blood clot formation, can significantly impact female fertility, particularly by affecting implantation and increasing the risk of pregnancy complications. Thrombophilia can be either inherited (genetic) or acquired, and it often involves abnormalities in the blood’s clotting mechanisms. While thrombophilia itself doesn’t directly prevent conception, it can create an environment that makes it difficult for a pregnancy to progress, leading to recurrent miscarriage, complications during pregnancy, and, in some cases, issues with implantation.
Types of Thrombophilia and Their Impact on Fertility
Thrombophilia can result from genetic mutations or acquired conditions that affect blood clotting. Common types of thrombophilia associated with infertility and pregnancy loss include:
Inherited Thrombophilias:
a) Factor V Leiden Mutation: This mutation makes the blood more prone to clotting by altering the function of clotting Factor V, a protein in the blood. Women with this mutation have an
b) Prothrombin Gene Mutation (G20210A): This mutation increases levels of prothrombin, another clotting protein, leading to a higher risk of blood clots and associated pregnancy loss.
c) Protein C, Protein S, and Antithrombin Deficiencies: These are rare inherited conditions that reduce the body’s ability to regulate clotting. Deficiencies in these proteins increase the risk of abnormal blood clots and complications in pregnancy.
Acquired Thrombophilia:
a) Antiphospholipid Syndrome (APS): APS is an autoimmune disorder where the immune system produces antibodies against phospholipids, which are molecules in cell membranes. APS is strongly associated with infertility and recurrent miscarriage, as the antibodies lead to blood clots in the placenta, reducing blood flow to the fetus and leading to pregnancy loss.
b) Hyperhomocysteinemia: Elevated levels of homocysteine (an amino acid) in the blood can increase the risk of blood clots and is associated with pregnancy loss and placental issues. Hyperhomocysteinemia is sometimes linked to MTHFR gene mutations.
How Thrombophilia Affects Implantation and Early Pregnancy
Blood clotting plays a critical role in early pregnancy, particularly in the establishment of the placenta, which supports the embryo. Thrombophilia can interfere with these processes in several ways.
Thrombophilia can cause microclots to form in the blood vessels of the uterus and placenta. These clots can restrict blood flow to the endometrium (uterine lining) and developing placenta, depriving the embryo of essential nutrients and oxygen. In the early stages of pregnancy, insufficient blood flow can prevent proper implantation and lead to early pregnancy loss.
Implantation, where the embryo attaches to the endometrial lining, requires a healthy blood supply and well-functioning blood vessels. If clotting disrupts blood flow to the uterus, it can create an inhospitable environment for the embryo, reducing the likelihood of successful implantation. Women with thrombophilia may experience unexplained infertility due to repeated implantation failure, even if fertilization occurs.
Thrombophilia is a well-known risk factor for recurrent miscarriage, defined as two or more consecutive pregnancy losses. Blood clots that form in the placental blood vessels can lead to placental insufficiency, where the placenta cannot adequately support the developing embryo or fetus. This can result in:
Early Pregnancy Loss: Many women with thrombophilia experience miscarriage within the first trimester, as blood clots form in the tiny blood vessels of the placenta, cutting off blood supply to the embryo.
Second-Trimester Loss: Thrombophilia can also lead to pregnancy loss in the second trimester due to poor placental development and blood flow, which can result in fetal growth restriction or placental abruption (where the placenta detaches from the uterine wall).
Stillbirth: In rare cases, thrombophilia may contribute to stillbirth if the blood flow to the fetus is severely restricted in the later stages of pregnancy.
Diagnostic Testing for Thrombophilia in Infertility
Women who experience recurrent pregnancy loss, unexplained infertility, or pregnancy complications may be tested for thrombophilia. Common tests include:
• Blood Clotting Tests: These tests measure levels of clotting factors and proteins in the blood, such as Factor V Leiden, prothrombin, protein C, protein S, and antithrombin.
• Antiphospholipid Antibody Test: This test checks for the presence of antiphospholipid antibodies, which are associated with APS. Testing may include anticardiolipin antibodies, lupus anticoagulant, and beta-2 glycoprotein I antibodies.
• Homocysteine Levels: Elevated homocysteine levels are associated with clotting disorders and may indicate an underlying genetic mutation, such as an MTHFR mutation.
With proper management, including anticoagulant therapy and close monitoring, many women with thrombophilia can successfully conceive and carry a healthy pregnancy to term. Early diagnosis and treatment are key to reducing the risks associated with thrombophilia and improving fertility outcomes.
8- Lifestyle Factors and Environmental Exposures
Lifestyle factors significantly impact female fertility by influencing ovulation, egg quality, hormone levels, and overall reproductive health. Several key lifestyle factors that can affect fertility include diet, weight, physical activity, stress levels, smoking, alcohol consumption, and exposure to environmental toxins.
1. Diet and Nutrition
A well-balanced diet that provides essential nutrients is vital for reproductive health. Specific nutrients, vitamins, and minerals support ovulation, hormone production, and egg quality. Poor nutrition or specific deficiencies can disrupt these processes, affecting fertility:
Antioxidants: Foods rich in antioxidants (such as vitamins C and E, folate, and beta-carotene) help protect eggs from oxidative stress, which can damage egg quality.
Folic Acid: This B-vitamin is crucial for DNA synthesis and cell division, making it essential for egg quality and embryo development.
Iron: Iron deficiency can lead to ovulatory issues, as iron is essential for blood production and oxygen transport.
Healthy Fats: Omega-3 fatty acids, found in fish, flaxseeds, and walnuts, help reduce inflammation and support hormone production. Trans fats, commonly found in processed foods, can negatively affect ovulation and should be avoided.
High Sugar and Processed Foods: Diets high in sugar and processed foods can lead to insulin resistance and hormonal imbalances, which can impact ovulation.
A healthy, nutrient-rich diet supports regular menstrual cycles, enhances egg quality, and promotes a favorable environment for conception.
2. Body Weight and Fertility
Body weight has a significant effect on fertility. Both being underweight or overweight can lead to hormonal imbalances that disrupt ovulation and menstrual regularity:
Underweight: Women with a very low body mass index (BMI) may experience irregular or absent periods (amenorrhea) due to low levels of estrogen. Estrogen is essential for ovulation and supporting a healthy menstrual cycle, and insufficient body fat can impair its production.
Overweight/Obesity: Excess weight, especially around the abdomen, is linked to hormonal imbalances and conditions like polycystic ovary syndrome (PCOS), which can hinder ovulation. Obesity can lead to insulin resistance, elevated androgen levels, and reduced fertility. Studies suggest that even a 5-10% weight reduction can improve ovulation and increase the likelihood of conception.
Maintaining a healthy weight is one of the most effective ways to optimize fertility and improve reproductive outcomes.
3. Physical Activity
Regular physical activity supports overall health, hormone regulation, and weight management. However, the impact of exercise on fertility can depend on its intensity:
• Moderate Exercise: Moderate, regular physical activity can improve fertility by regulating weight, reducing stress, and enhancing blood flow to reproductive organs. It’s generally recommended for women trying to conceive.
• Excessive Exercise: Intensive, high-impact exercise can lead to hormonal imbalances, especially in women with low body fat. Excessive exercise can cause a decrease in estrogen levels, disrupt menstrual cycles, and, in severe cases, lead to hypothalamic amenorrhea (absence of periods due to insufficient hormone production).
Balancing physical activity levels is key to supporting reproductive health, with a focus on moderate and consistent exercise routines.
4. Stress and Emotional Well-Being
Chronic stress can negatively affect fertility by disrupting hormonal balance and ovulation. High levels of stress increase the production of cortisol, a hormone that can interfere with reproductive hormones like GnRH (gonadotropin-releasing hormone), which regulates ovulation. Stress is also associated with unhealthy coping behaviors, such as poor diet, lack of sleep, and physical inactivity, which can further impact fertility. Additionally, the emotional toll of stress can make conception more challenging and may even contribute to a cycle of stress and infertility. Mindfulness practices, counseling, and stress management techniques such as yoga, meditation, or therapy can be beneficial for women trying to conceive.
5. Smoking
Smoking is one of the most harmful lifestyle factors affecting fertility. Tobacco contains toxic chemicals, such as nicotine, carbon monoxide, and tar, which can impact reproductive health in multiple ways:
• Egg Quality: Smoking accelerates the loss of eggs and damages their quality, leading to a higher risk of chromosomal abnormalities.
• Ovulation: Smokers are more likely to experience ovulatory issues, making it more difficult to conceive.
• Uterine Health: Smoking affects blood flow to the uterus, potentially creating an unfavorable environment for embryo implantation.
• Early Menopause: Women who smoke often reach menopause 1-4 years earlier than non-smokers, reducing their fertile window.
Even exposure to secondhand smoke can impact fertility. Quitting smoking is one of the most beneficial changes a woman can make to improve her chances of conceiving.
6. Alcohol Consumption
Excessive alcohol consumption can harm fertility and pregnancy outcomes:
• Ovulation and Hormonal Imbalances: Heavy alcohol intake can interfere with the production of hormones necessary for ovulation and menstrual regularity, increasing the risk of infertility.
• Reduced Fertility Rates: Studies suggest that women who consume high levels of alcohol may experience reduced fertility rates compared to moderate or non-drinkers.
• Risks During Pregnancy: Drinking alcohol can affect early pregnancy, increasing the risk of miscarriage and fetal developmental issues.
While moderate alcohol intake may have minimal effects, abstaining or reducing alcohol consumption is generally recommended for women trying to conceive.
7. Caffeine Intake
While moderate caffeine intake (e.g., up to 200 mg per day, or about one 12-ounce coffee) is generally considered safe, high levels of caffeine consumption may negatively impact fertility:
• Delayed Conception: Some studies have shown that high caffeine intake may be associated with a delay in conception or an increased risk of miscarriage.
• Reduced Absorption of Certain Nutrients: Excessive caffeine can interfere with the absorption of essential nutrients like calcium and iron, which are important for reproductive health.
Most health professionals recommend limiting caffeine intake when trying to conceive.
8. Environmental Toxins and Chemical Exposure
Exposure to environmental toxins, including certain chemicals, pollutants, and endocrine-disrupting chemicals, can impact fertility:
• Endocrine Disruptors: Chemicals like bisphenol A (BPA), phthalates, and pesticides can mimic or block natural hormones, leading to hormonal imbalances that disrupt ovulation, menstrual cycles, and overall reproductive function.
• Heavy Metals: Exposure to heavy metals like lead and mercury can impair ovarian function, reduce egg quality, and interfere with the menstrual cycle.
• Occupational Hazards: Women working in certain industries, such as agriculture, manufacturing, or laboratories, may be exposed to chemicals and radiation that negatively affect fertility.
Reducing exposure to environmental toxins—such as by avoiding plastic containers with BPA, choosing organic foods when possible, and minimizing use of certain cosmetics—can help support reproductive health.
9. Sleep Patterns and Circadian Rhythm
Poor sleep habits and irregular sleep patterns can affect fertility by disrupting hormone levels, particularly the reproductive hormones that regulate ovulation and menstrual cycles:
• Melatonin and Reproductive Health: Melatonin, a hormone associated with sleep, also acts as an antioxidant that protects eggs from damage. Women with insufficient or poor-quality sleep may experience reduced melatonin levels, potentially impacting egg health.
• Shift Work: Women who work night shifts or have irregular hours may experience disrupted circadian rhythms, which can lead to menstrual irregularities and reduced fertility.
Maintaining consistent sleep patterns and aiming for 7-8 hours of sleep per night can help support hormonal balance and reproductive health.
10. Recreational Drug Use
Use of recreational drugs, such as marijuana, cocaine, and other substances, can have harmful effects on fertility:
• Hormonal Imbalances: Many recreational drugs interfere with the hormonal signals necessary for ovulation and menstrual regularity.
• Egg Quality and Ovulation: Certain drugs, particularly marijuana, have been linked to disruptions in ovulation and reduced egg quality.
• Pregnancy Complications: Drug use during conception or pregnancy can lead to miscarriage, birth defects, and developmental issues in the fetus.
Avoiding recreational drugs is essential for optimizing fertility and reducing risks associated with pregnancy.
North Cyprus IVF Centre
North Cyprus IVF Centre is a patient focused fertility clinic, located within “Elite Research and Surgical Hospital” in Nicosia, Cyprus. Our clinic is one of the most advanced fertility clinics in the world, offering a wider selection of treatment options at affordable prices.
Contact Us
Phone
English: +90 548 875 8000
French: +90 548 876 8000
Turkish: +90 542 869 8000
Arabic: +90 548 875 8000
German: +90 548 830 1987
Russian: +90 548 828 9955
info@northcyprusivf.net
© 2020 LowCostIVF - All rights reserved. Privacy Policy

