- About
- Blog
- Infertility
- IVF Treatments
- New IVF Advances
- Mitochondrial Replacement Therapy!
- IVF Treatment in Cyprus
- IUI
- Mini IVF
- IVF + ICSI
- Cytoplasmic IVF
- Tandem IVF Cycle
- Egg Donation
- Embryo Donation
- Sperm Donation
- Gender – Sex Selection
- Gestational Surrogacy
- Same Sex Gay Surrogacy
- Surgical Sperm Retrieval
- Pre Implantation Genetic Diagnosis PGD
- Egg Freezing
- Gender Selection Using Donor Eggs
- Sickle Cell Disease Prevention
- F.A.Q.
- Travel
- About
- Blog
- Infertility
- IVF Treatments
- New IVF Advances
- Mitochondrial Replacement Therapy!
- IVF Treatment in Cyprus
- IUI
- Mini IVF
- IVF + ICSI
- Cytoplasmic IVF
- Tandem IVF Cycle
- Egg Donation
- Embryo Donation
- Sperm Donation
- Gender – Sex Selection
- Gestational Surrogacy
- Same Sex Gay Surrogacy
- Surgical Sperm Retrieval
- Pre Implantation Genetic Diagnosis PGD
- Egg Freezing
- Gender Selection Using Donor Eggs
- Sickle Cell Disease Prevention
- F.A.Q.
- Travel
Stem Cell Therapy For Azoospermia
Azoospermia Treatment
How Can Stem Cell Treatment Help With Male Infertility?
Azoospermia is a condition where there are no live or mature sperm cells in a man’s ejaculate. In most cases, ejaculate may contain semen with other cells, therefore, existence of a fluid ejaculate does not suggest existence of sperm cells. Azoospermia can be divided into two main categories:
Obstructive azoospermia: When an obstruction occurs, sperm cells cannot be released into the ejaculate, even though sperm production remains active. Obstructive azoospermia can result from various causes. The most common cause today is vasectomy, a condition patients are typically aware of. However, other factors can also block sperm from entering the ejaculate. These factors include:
1. Ductal System Abnormalities
Sperm cells must travel through the ductal system to reach the urethra. After being produced in the testes (testicles), sperm passes through several structures, including the epididymis, vas deferens (or ductus deferens), ejaculatory duct, and urethra. Any disruption in the transport of sperm through or between these structures can obstruct its pathway. (Refer to the figure below for a visual representation of these structures.)
2. Ejaculatory Dysfunction
The process of emission, in which sperm is deposited into the urethra prior to ejaculation, can malfunction. This dysfunction may result from neurological damage or, in some cases, uncontrolled diabetes.
In cases of obstructive azoospermia, using surgical sperm extraction techniques often help obtain an acceptable number of viable sperm cells to be used during an IVF cycle.
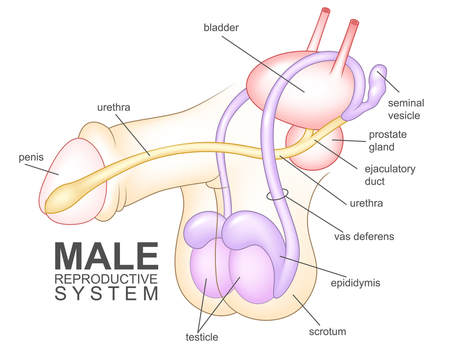
Non-obstructive azoospermia treatment: Non-obstructive azoospermia (NOA) is a condition characterized by the absence of sperm in the ejaculate due to impaired sperm production within the testes. Unlike obstructive azoospermia, which results from blockages in the reproductive tract, NOA arises from intrinsic testicular dysfunction. This can occur due to genetic abnormalities (such as Klinefelter syndrome or Y-chromosome microdeletions), hormonal imbalances, or damage to the testes from infections, trauma, chemotherapy, or radiation. In many cases, the underlying cause remains idiopathic. NOA is a significant cause of male infertility and often presents with smaller testicular volume and elevated levels of follicle-stimulating hormone (FSH) as the body attempts to compensate for reduced spermatogenesis. Diagnosis typically involves hormonal profiling, genetic testing, and occasionally testicular biopsy to confirm the absence of mature sperm.
The purpose of a testicular biopsy is to thoroughly inspect the testicular tissue for the presence of less mature sperm cells. Even when spermatogenesis (the process of sperm production and maturation) doesn’t go to full completion, there might be earlier stages of sperm cells present which can possibly be used. The following diagram shows the complete cycle of spermatogenesis from stem cells to mature sperm cells:
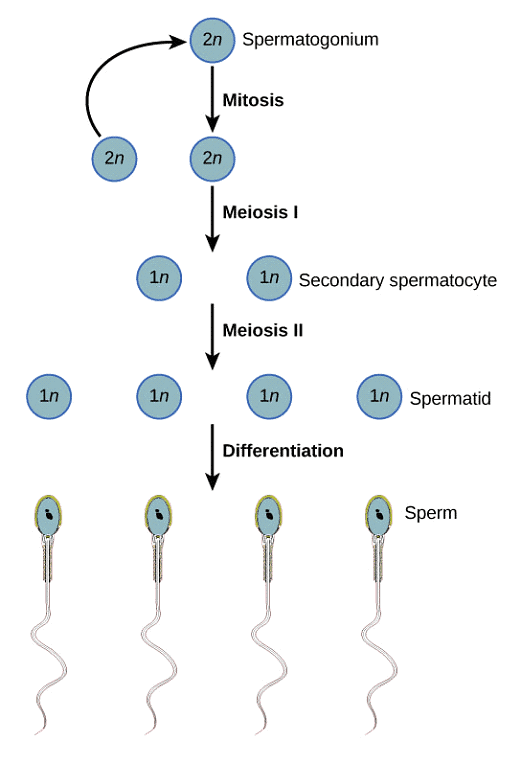
If the patient’s biopsy reveals presence of late stage spermatids (elongated spermatids), even though natural conception will not work, late stage spermatids are often successfully used during an IVF cycle with the use of ICSI. However, any stage of sperm maturation before elongated spermatids will not result in a successful IVF outcome.
At this point, if there are no germ cells present and the diagnosis of sertoli cell only syndrome is made, then the only other option for fathering a child will be through the use of donor sperm or adoption. Germ cells are immature sperm cells that have the potential to grow and differentiate into mature sperm cells. These can be considered as immature and undifferentiated sperm cells. They include:
– Spermatogonia
– Primary spermatocytes
– Secondary spermatocytes
– Early stage spermatids
– Late stage spermatids
Presence of germ cells in the testicular tissue is a prerequisite for stem cell therapy. The reason is simple. We use mesenchymal stem cells from the patient’s own adipose tissue for stem cell treatment. Mesenchymal stem cells have limited differentiation potential. However, given the right conditions, they can differentiate into many cell types. Mesenchymal stem cells mainly tend to differentiate into surrounding cell types unless they are specifically programmed to differentiate into a specific cell type. In our treatments, there is no cell programming involved. The patient’s own adipose tissue derived stem cells are isolated and they are injected into the testicular tissue where germ cells are present. Having germ cells in the surrounding, stem cells follow them as a template and they also differentiate into germ cells. However, this is only one part of the process. Given that existing germ cells in the patient’s testes are unable to complete spermatogenesis and become fully mature sperm cells, there need to be additional steps to promote such differentiation.
When we introduce stem cells into the seminiferous tubules, we also inject the patient’s own platelet rich plasma that is derived from his peripheral blood sample. Platelet rich plasma is rich in growth factors that allow cellular growth and development as well as improving blood supply to the tissue. Therefore, stem cells enjoy a rich infusion of growth factors into the vicinity in order to facilitate their development. Furthermore, we use placenta-derived exosomes in order to enhance cellular communication in the testes.
Stem cell therapy, in its entirety, aims to:
1- Increase the population of germ cells in the testicular tissue by providing stem cells into the testicular tissue.
2- Allow these germ cells to grow and develop with the aid of growth factors.
3- Improve cellular communication and facilitate response to hormonal stimulation with the aid of exosomes.
What is Platelet-Rich Plasma (PRP)? PRP is a concentration of platelets derived from an individual’s own blood, enriched with growth factors and bioactive proteins that promote healing and tissue regeneration. PRP is commonly used in medical and aesthetic treatments to accelerate the repair of damaged tissues, reduce inflammation, and stimulate cell proliferation.
Platelets play a key role in wound healing and tissue repair by releasing growth factors such as:
⦁ Platelet-Derived Growth Factor (PDGF): Stimulates cell growth and new blood vessel formation.
⦁ Transforming Growth Factor-Beta (TGF-β): Promotes tissue repair and reduces inflammation.
⦁ Vascular Endothelial Growth Factor (VEGF): Encourages the formation of new blood vessels.
⦁ Epidermal Growth Factor (EGF): Supports cell regeneration and wound healing.
When PRP is injected into a target area, these growth factors recruit and activate the body’s natural healing processes, including stem cell activation and collagen synthesis.
What are Exosomes?
Exosomes are tiny nanovesicles found in interstitial spaces as well as bodily fluids. Due to their sizes, exosomes have always been viewed as artifacts or cellular trash with no physiological function. However, with advancements in research in the field of medicine, they are now known to be a part of a meticulous intracellular communication system.
Exosomes are known for their involvement in all types of cellular communication. They provide local autocrinesignals between similar types of cells, local paracrine signals between different cell types as well as distant endocrine signals. Involvement in such extensive cellular signaling has gained them the name “signalosomes”. When exosomes are introduced in the body, they enhance communication via all channels, which helps with the overall health and function of cells.
Compared to adult stem cells, exosomes contain 300% more growth factors, which are essential for cellular growth, development and regeneration. Exosomes can be given via IV infusion or can be directed at a specific site by direct injection.
How is Stem Cell Therapy Carried Out?
At North Cyprus IVF Center, the stem cell therapies we carry out for non-obstructive azoospermia treatment utilize a cocktail of mesenchymal stem cells, platelet rich plasma and exosomes. Stem cell isolation requires a surgical procedure called liposuction.
The liposuction procedure for obtaining mesenchymal stem cells (MSCs) is a minimally invasive surgical technique used to harvest adipose tissue, which is a rich source of MSCs. The surgical procedure involves a number of steps:
1. Preoperative Preparation:
The patient undergoes a medical evaluation to ensure they are suitable for the procedure. This involves a series of hormone testing, potentially an evaluation of the scrotum and a testicular biopsy to assess the cellularity of the seminiferous tubules.
The patient is also asked to perform a number of blood tests to check liver and kidney function as well as certain tumor murkers such as Alpha-fetoprotein (AFP), and beta human-chorionic gonadotropin (b-hCG).
2. Tumescent Solution Injection:
A tumescent solution, containing saline, lidocaine (a local anesthetic), and epinephrine (to reduce bleeding), is injected into the targeted area. This solution helps loosen fat cells, reduces pain, and minimizes bleeding during the procedure.
3. Fat Extraction (Liposuction):
A small incision is made in the skin, and a cannula (a thin, hollow tube) is inserted. The cannula is connected to a vacuum device, which gently suctions fat tissue from areas such as the abdomen, thighs, or buttocks.
4. Processing the Adipose Tissue:
The extracted fat is collected into sterile containers and processed to isolate MSCs. Processing involves:
Centrifugation to separate fat from other components like blood and fluids,
Enzymatic digestion using collagenase to break down extracellular matrix and release MSCs.
Filtration and Washing to purify the MSCs and remove debris.
5. Reimplantation:
The MSCs are made into a cocktail with PRP and exosomes and are directly injected into target areas in the testes. The whole procedure is done within a few hours when the patient is under sedation. Therefore, the liposuction and reimplantation procedures are carried out in one go without necessitating two separate sedations.
While the procedure is generally safe, risks include minor bruising, infection, or discomfort at the liposuction or reimplantation site. This technique is widely used in regenerative medicine due to the high potency and versatility of adipose-derived MSCs.
Required Length of Stay in Cyprus:
Majority of our patients travel to us from abroad. Due to commitments at work or home, travel times can be limited. This is why we ask for the preliminary tests/ biopsy to be performed locally prior to the patient’s arrival so that the length of stay in Cyprus can be minimized. You will be expected to stay in Cyprus for approximately 3 days for the stem cell therapy. This will give us enough time to run the necessary pre-operative tests, obtain your blood sample and perform the procedure. You will be expected to stay overnight at our hospital for observation and will be able to travel back home the next day.
What to Expect After the Procedure? Success Rates:
After injection with stem cells, there is a three month waiting period to observe the effect. Spermatogenesis (production and maturation of sperm cells) takes about 65 to 70 days. Therefore, stem cell that are implanted will require this length of time before we can observe any effects.
Success of the procedure largely depends on the baseline cellularity of the testicular tissue to begin with. Patients who have already have early to late stage spermatids in their testicular tissue (Johnsen score of 7 or 8) are the group of patients who have the highest chance of success with stem cell therapy. In most cases, we are able to observe mature sperm cells in the ejaculate after the three month period. While the number of mature sperm cells in the ejaculate will not be sufficient for natural conception, we often plan and execute a successful IVF cycle with this group of patients. The chance of successfully obtaining mature sperm cells in the ejaculate in this group of patients is around 75%.
Patients who do not have any spermatids in the seminiferous tubules (maybe possibly a few) but have secondary spermatocytes (Johnsen score of 5 to 6) are also like to see an improvement in their spermatogenic activity that can result in mature sperm cells that can enter the ejaculate. However, in this group of patients, observing mature sperm cells in the ejaculate is less common. Our expectation in this group of patients is to be able to find maturing spermatids in a testicular biopsy to be used during an IVF cycle. If the semen analysis performed three months after the stem cell therapy does not indicate any sperm cells in the ejaculate, then the next step will be to organize a testicular sperm extraction procedure in order to obtain viable sperm cells that can be used during ICSI. The chance of successfully obtaining mature sperm cells in the ejaculate in this group of patients is around 25%. The chance of finding viable sperm cells either in the ejaculate or via TESE is around 40%.
Patients who only have primary spermatocytes in their seminiferous tubules (Johnsen score of 4) are not likely to observe mature sperm cells in the ejaculate after stem cell procedure. In majority of the cases, we see an improvement in spermatogenic activity in the sense that tissue becomes more populated with germ cells. However, in a great majority of patients, we do not find sufficiently mature sperm cells to be used during ICSI. Success with this group of patients can be estimated at 15%, meaning, 15% of patients will have mature enough sperm cells which can be used in an IVF cycle.
Based on our patient statistics to date, patients with only spermatogonia in the seminiferous tubules (Johnsen score of 3) are likely to have a chance of 6% of having mature enough sperm cells that can be used during ICSI after the stem cell proedure.
We do not expect a successful outcome in group of patients who have a Johnsen score of 2 or below (Sertoli cell only syndrome or no cells).
What is a Johnsen Score?
The Johnsen Scoring System is a histological method used to evaluate testicular biopsy specimens by assessing spermatogenesis (the production of sperm) in seminiferous tubules. Developed by S.G. Johnsen in 1970, the system assigns a numerical score to each seminiferous tubule based on the presence and maturity of germ cells, as well as the overall state of spermatogenesis. It is commonly used in the evaluation of male infertility.
Scoring Criteria
Each seminiferous tubule is assigned a score ranging from 1 to 10 based on the following criteria:
Score 10: Full spermatogenesis with many spermatozoa visible.
Score 9: Slightly reduced spermatogenesis but with many late-stage spermatids present.
Score 8: Few late spermatids, indicating moderately impaired spermatogenesis.
Score 7: Presence of many early spermatids but no late spermatids or spermatozoa.
Score 6: Presence of few early spermatids but no late spermatids or spermatozoa.
Score 5: Many spermatocytes present, but no spermatids or spermatozoa.
Score 4: Few spermatocytes present, but no spermatids or spermatozoa.
Score 3: Spermatogonia (germ cell precursors) present but no spermatocytes, spermatids, or spermatozoa.
Score 2: Sertoli cells only with no germ cells present.
Score 1: No seminiferous tubules present in the biopsy (extreme testicular damage).
Interpretation of Scores
A higher score indicates normal or near-normal spermatogenesis.
A lower score reflects impaired or absent spermatogenesis.
By calculating the mean Johnsen score across all tubules examined, we can quantify the degree of spermatogenic impairment and make informed decisions about treatment options, such as moving forward with assisted reproductive technologies, using stem cell treatments or considering donor options.
Cost of Stem Cell Therapy for Non-Obstructive Azoospermia:
The cost of stem cell therapy for the treatment of non-obstructive azoospermia is 7,000 Euros, including the PRP protocol, liposuction and stem cell purification, exosomes and implantation into the testicular tissue. An overnight stay at the hospital is also included in this quote.
Please feel free to contact us for more information.
New Advances in IVF Technologies
North Cyprus IVF Centre
North Cyprus IVF Centre is a patient focused fertility clinic, located within “Elite Research and Surgical Hospital” in Nicosia, Cyprus. Our clinic is one of the most advanced fertility clinics in the world, offering a wider selection of treatment options at affordable prices.
Contact Us
Phone
English: +90 548 875 8000
French: +90 548 876 8000
Turkish: +90 542 869 8000
Arabic: +90 548 875 8000
German: +90 548 830 1987
Russian: +90 548 828 9955
info@northcyprusivf.net
© 2020 LowCostIVF - All rights reserved. Privacy Policy


