- معلومات عنا
- مدونة
- العقم
- علاجات أطفال الأنابيب
- التطورات الجديدة في عمليات التلقيح الصناعي
- العلاج باستبدال الميتوكوندريا!
- علاج أطفال الأنابيب في قبرص
- IUI
- التلقيح الاصطناعي المصغر
- أطفال الأنابيب + الحقن المجهري
- التلقيح الاصطناعي السيتوبلازمي
- دورة التلقيح الاصطناعي الترادفية
- التبرع بالبويضات
- التبرع بالجنين
- التبرع بالحيوانات المنوية
- الجنس - اختيار الجنس
- تأجير الأرحام الحملي
- تأجير الأرحام مثلي الجنس من نفس الجنس
- سحب الحيوانات المنوية جراحيًا
- التشخيص الجيني قبل الزرع PGD
- تجميد البويضات
- اختيار الجنس باستخدام بيض المتبرع
- الوقاية من مرض فقر الدم المنجلي
- التعليمات
- يسافر
- معلومات عنا
- مدونة
- العقم
- علاجات أطفال الأنابيب
- التطورات الجديدة في عمليات التلقيح الصناعي
- العلاج باستبدال الميتوكوندريا!
- علاج أطفال الأنابيب في قبرص
- IUI
- التلقيح الاصطناعي المصغر
- أطفال الأنابيب + الحقن المجهري
- التلقيح الاصطناعي السيتوبلازمي
- دورة التلقيح الاصطناعي الترادفية
- التبرع بالبويضات
- التبرع بالجنين
- التبرع بالحيوانات المنوية
- الجنس - اختيار الجنس
- تأجير الأرحام الحملي
- تأجير الأرحام مثلي الجنس من نفس الجنس
- سحب الحيوانات المنوية جراحيًا
- التشخيص الجيني قبل الزرع PGD
- تجميد البويضات
- اختيار الجنس باستخدام بيض المتبرع
- الوقاية من مرض فقر الدم المنجلي
- التعليمات
- يسافر
العلاج باستبدال الميتوكوندريا
تقنية طبية جديدة رائدة
Mitochondrial Replacement Therapy (MRT) at North Cyprus IVF Center
In collaboration with Dr. Shoukhrat Mitalipov – Pioneering the Future of Fertility
At North Cyprus IVF Center, we are proud to collaborate with Dr. Shoukhrat Mitalipov, one of the world’s foremost experts in reproductive genetics and mitochondrial biology. Dr. Mitalipov is internationally recognized for developing Mitochondrial Replacement Therapy (MRT)—a cutting-edge technique originally designed to prevent the transmission of mitochondrial disease, now offering renewed hope to women with age-related infertility and recurrent IVF failure.
This partnership allows us to offer MRT as a specialized fertility treatment outside of a clinical trial setting, combining the expertise of Dr. Mitalipov’s team with the clinical experience of Dr. Savas Ozyigit, Dr. Ahmet Ozyigit and the embryology team at North Cyprus IVF Center, located at Elite Hospital.
What Are Mitochondria?
Mitochondria are the energy-producing organelles found in nearly every cell in the body, including the oocytes (eggs). As a woman ages, mitochondrial number and function decline, impairing the oocyte’s ability to support normal fertilization, embryo development, and implantation. This decline is one of the major contributors to age-related infertility.
The Role of Mitochondria in Cellular and Reproductive Health
Mitochondria are double-membraned organelles best known as the cell’s “powerhouses” because they generate adenosine triphosphate (ATP), the primary energy currency used to fuel nearly all cellular processes. However, mitochondria also play crucial roles in:
• Regulating apoptosis (programmed cell death)
• Calcium homeostasis
• Reactive oxygen species (ROS) management
• Steroidogenesis and cell signaling
• Oocyte maturation, spindle formation, and early embryonic development
In human oocytes, each cell contains between 100,000 to 600,000 mitochondria—many more than typical somatic cells—underscoring their central role in reproductive competence (May-Panloup et al., 2005).
As women age, particularly after the age of 35, mitochondrial function in oocytes undergoes progressive deterioration:
1. Reduced mtDNA Copy Number
Oocyte mitochondria rely on sufficient mitochondrial DNA (mtDNA) copies to maintain ATP production during maturation and fertilization. Studies show a significant reduction in mtDNA copy number with age, compromising the energy availability necessary for normal spindle formation and chromosomal segregation (Fragouli et al., 2015).
2. Increased Mitochondrial DNA Mutations
mtDNA lacks protective histones and has limited repair mechanisms, making it highly susceptible to oxidative damage. With age, cumulative exposure to ROS leads to increased mtDNA mutations, which impair mitochondrial respiration and further exacerbate oxidative stress (Barritt et al., 2002; Wallace, 2013).
3. Impaired ATP Production
Aged mitochondria generate less ATP through oxidative phosphorylation, resulting in energy-deficient oocytes that struggle to complete meiotic division or support early embryogenesis (Bentov & Casper, 2013).
4. Altered Membrane Potential and Fragmentation
Aged mitochondria exhibit loss of membrane potential, increased fragmentation, and altered morphology, leading to impaired cellular metabolism and apoptosis susceptibility (Wilding et al., 2001).
5. Association with Aneuploidy and Poor Embryo Quality
Mitochondrial dysfunction in oocytes has been correlated with spindle abnormalities, chromosomal missegregation, and lower blastocyst formation rates (Santos et al., 2006). Mitochondrial dysfunction in oocytes has been shown to impair spindle assembly and chromosomal segregation, leading to meiotic errors and compromised embryo development—a phenomenon demonstrated in both human and non-human primate models by Dr. Shoukhrat Mitalipov and colleagues (Tachibana et al., 2009, Nature).
How does Mitochondrial Replacement Therapy fit in?
Mitochondrial health is not just a marker of oocyte vitality—it is a limiting factor in reproductive success. Mitochondrial replacement therapy aims to restore a bioenergetically competent cytoplasm, allowing the patient’s nuclear DNA to function in a rejuvenated cellular environment.
By replacing aged, dysfunctional mitochondria with healthy donor mitochondria, MRT addresses a root cause of age-related infertility at the cellular level—something traditional IVF cannot overcome when the egg’s cytoplasmic environment is compromised.
MRT is a revolutionary technique that replaces the dysfunctional mitochondria in a woman’s egg with healthy mitochondria from a donor egg, creating a more viable environment for embryo development—while preserving the woman’s nuclear genetic material.
How MRT Works?
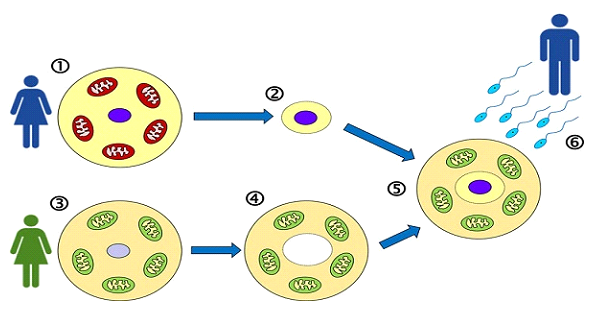
Unlike cytoplasmic transfer—where a small amount of donor egg cytoplasm is injected into a patient’s egg—MRT involves the complete transfer of the mother’s nuclear DNA into an enucleated donor egg that retains its own healthy mitochondria. This technique is known as Maternal Spindle Transfer (MST) and it allows for precise visualization and manipulation of the genetic material.
Steps of the MRT process:
• Egg Retrieval: The patient undergoes ovarian stimulation and egg collection. Due to lower egg yield in women over 40, multiple retrieval cycles may be necessary. A minimum of 3 M2 grade oocytes is required for MRT process. Ther more oocytes a patient has, the higher the chances of success. Patient’s eggs are frozen until a suitable egg donor is found and her eggs are ready to be retrieved.
• Donor Egg Preparation: A donor egg is retrieved and its nucleus is carefully removed, leaving behind cytoplasm with intact, healthy mitochondria. This is a procedure done on fresh oocytes. Therefore, the MRT protocol is done with fresh egg donor oocytes only.
• Spindle Transfer: The patient’s nuclear DNA (genetic material) is extracted and transferred into the enucleated donor egg.
• Fertilization: The reconstructed egg—now containing the patient’s genetic material and the donor’s healthy mitochondria—is fertilized using ICSI (intracytoplasmic sperm injection). In most cases, providing a fresh sperm sample may not be a possibility if patients are traveling from abroad. Therefore, we ask for a sperm sample to be provided beforehand and be kept frozen in preparation for an MRT cycle.
• Embryo Development: Resulting embryos are cultured to the blastocyst stage and then vitrified (frozen) for future transfer. Because the donor oocytes are used fresh, it would technically be the first time freezing would be attempted (even though the patient’s oocytes were previously frozen)
• Embryo Transfer: In a subsequent cycle, the patient undergoes endometrial preparation for embryo transfer.
Mitochondrial replacement therapy (MRT) involves transferring nuclear genetic material from a woman’s egg into a donor egg with healthy mitochondria. The goal is to preserve the patient’s nuclear DNA while providing a bioenergetically competent cytoplasm. There are two principal techniques used to achieve this:
1. Maternal Spindle Transfer (MST)- The method used at our IVF unit.
MST is performed at the metaphase II (MII) oocyte stage, prior to fertilization. The maternal meiotic spindle complex (containing chromosomes) is carefully removed from the patient’s unfertilized egg and transferred into a donor egg that has had its own spindle removed. The reconstructed egg is then fertilized with sperm.
This method is ideal for infertility treatment and age-related oocyte dysfunction, as it avoids post-fertilization manipulation. Mitochondrial Carryover risk is typically <1%, minimizing the risk of mitochondrial disease recurrence if the indication for MRT is mitochondrial disease. If not, this is rather irrelevant.
2. Pronuclear Transfer (PNT)
PNT is performed at the zygote stage, after fertilization has occurred. The pronuclei (male and female) are removed from a fertilized egg and transferred into a donor zygote that has been enucleated. This method is mainly studied for preventing inherited mitochondrial diseases. However, the risk of mitochondrial carryover is higher than MST (~2–5%), depending on technique.
Why Spindle Transfer Is Superior in Fertility Applications?
Ethical and Legal Considerations
MST avoids creating and destroying embryos, as it takes place before fertilization. This has made it more acceptable ethically and legally in many jurisdictions compared to PNT. PNT involves manipulating a fertilized zygote, raising more significant bioethical concerns.
Lower Mitochondrial Carryover
MST consistently demonstrates lower mtDNA carryover, reducing the risk of transmitting mitochondrial mutations to the offspring. Mitalipov et al. (2009, 2016) showed that MST resulted in undetectable or negligible mtDNA from the original oocyte, while PNT may carry higher amounts of defective mtDNA due to cytoplasmic mixing.
Reduced Risk of Epigenetic Reprogramming Issues
Because MST occurs before fertilization, epigenetic remodeling takes place within a single cytoplasmic environment, potentially resulting in more stable gene expression during embryogenesis. In contrast, PNT combines already-fertilized nuclei with a foreign cytoplasm, which may lead to epigenetic discordance or delayed reprogramming.
Better Embryo Development Outcomes
In studies on human oocytes and embryos, including those from Tachibana et al. (2013) and Zhang et al. (2017), MST embryos showed higher developmental competence and lower fragmentation rates than PNT-derived embryos. MST has also been more readily translated into fertility applications, as seen in the first live birth via MST in Greece (2019), involving a patient with repeated IVF failures.
Who Is a Candidate for MRT?
MRT is considered in the following clinical scenarios:
• Women with documented mitochondrial dysfunction or markers suggestive of poor oocyte cytoplasmic quality
• Patients in advanced maternal age (typically >40) with repeated IVF failures due to oocyte-related factors
• Women with normal ovarian reserve but poor embryo development or fertilization rates suggestive of cytoplasmic insufficiency
• Individuals seeking a biologically related child but previously advised to consider egg donation due to poor oocyte quality
Scientific Background and Clinical Results:
In 2019, MRT was successfully used to achieve the birth of a healthy child in Greece through a collaboration led by Dr. Mitalipov. The mother had suffered multiple failed IVF cycles due to poor egg quality. This case marked one of the first live births using Maternal Spindle Transfer for infertility rather than genetic disease prevention.
A reported success rate of 25% was observed in this cohort—meaning 1 in 4 women who previously failed IVF achieved pregnancy using MRT. While this number may seem modest, it is significant for women previously considered non-responders to standard IVF protocols.
We should keep in mind that although women who took part in this study had multiple failed IVF cycles, they were aged 40 and below. The 25% rate may not apply to women in more advanced age brackets. However, women in the Greece trial underwent a single oocyte collection procedure and in most cases, they had very limited number of oocytes. At North Cyprus IVF Center, we usually aim to obtain as many oocytes as possible through multiple egg collections, especially in patients who are over the age of 43 in order to improve odds.
The Treatment Process at North Cyprus IVF Center
Because egg quantity and quality tend to decline together in women of advanced age, most candidates will require two or more egg retrieval cycles to accumulate a sufficient number of mature oocytes for MRT. Here’s how the process unfolds:
1. Ovarian Stimulation and Egg Freezing
Each retrieval cycle involves stimulation and egg collection, followed by vitrification of the eggs. This can be repeated as needed (typically 2–3 times) depending on the ovarian response. We have patients who obtain 9-10 oocytes in one cycle and we also have patients who go through 4-5 oocyte retrievals before an ideal number of oocytes are obtained. Some patients prefer to have as many as 15-16 oocytes in order to have multiple embryos that may potentially allow for a future sibling project.
2. Fresh Donor Egg Coordination
Once a sufficient number of the patient’s eggs have been frozen, fresh donor oocytes are arranged to coincide with the MRT procedure. Fresh eggs are essential as mitochondrial integrity and cytoplasmic activity degrade with freezing.
3. MRT & Embryo Creation
The MRT procedure is performed using the maternal spindle transfer technique, followed by ICSI to fertilize the reconstructed oocytes. Embryos are cultured to the blastocyst stage and frozen for future transfer. Dr. Shoukhrat Mitalipov usually travels to Cyprus twice a year (once in July and once in December) and this is when the procedures are expected to be performed. Depending on patient volume, a third MRT procedure can be added to the schedule.
4. Endometrial Preparation and Embryo Transfer
In a separate cycle, the patient’s uterus is prepared hormonally to receive the embryo. A frozen embryo transfer (FET) is then performed once endometrial thickness and receptivity are confirmed.
Timeline & Logistics for International Patients
Most of our MRT patients travel from abroad, and we accommodate flexible scheduling by coordinating initial work-up remotely:
• Ovarian stimulation and preliminary monitoring (hormones, scans) can be done locally under our guidance.
• You will only need to travel to Cyprus for each egg retrieval, typically requiring a 3–5 day stay.
• After MRT is completed and embryos are frozen, a separate trip of 2 days is required for the final embryo transfer.
Cost and Planning
The total cost of MRT treatment depends on how many egg retrieval cycles are needed. On average, patients undergo two egg collections, bringing the overall cost to approximately €14,000. This includes:
• Two egg retrievals and medication protocols
• Vitrification and storage of oocytes
• Fresh donor egg procurement
• MRT procedure (spindle transfer and embryo creation)
• Blastocyst culture and freezing
• Final frozen embryo transfer with endometrial preparation
Each additional oocyte retrieval procedure carries a cost of €2,500. In some cases, patients often choose to have ovarian PRP and exosome treatment performed in between cycles in order to imrpove oocyte count and quality for a subsequent oocyte collection.
Is Haplogroup Matching a Concern?
At present, we do not perform mitochondrial donor selection based on maternal haplogroup matching. While the concept of haplogroup compatibility has been discussed in the scientific literature, the clinical relevance of mito-nuclear mismatch remains theoretical and has not been shown to impact embryo development, implantation, or early postnatal health in human MRT cases. In both human and non-human primate models—including those performed by Dr. Mitalipov’s team—haplogroup mismatching has not resulted in adverse developmental outcomes (Tachibana et al., 2009, Nature; Zhang et al., 2017). While long-term, multi-generational tracking of mito-nuclear interactions in humans is still ongoing, current evidence suggests that short- to mid-term developmental outcomes are not compromised by haplogroup mismatches (Mitalipov & Wolf, Nat Rev Mol Cell Biol, 2014).
Moreover, haplogroup testing is not a standard, validated screening test in egg donor programs and is not routinely available for clinical application. Our primary focus is on selecting donors with excellent oocyte quality, healthy mitochondrial function, and clinical screening for genetic and infectious diseases, which are more impactful on treatment outcomes.
Studies, including those led by Dr. Mitalipov, have shown that embryos created using non-matched mitochondrial haplogroups (e.g., from different ethnic backgrounds or lineages) can develop normally and result in healthy births.
Why Choose MRT Over Egg Donation?
MRT offers a unique opportunity for women to conceive using their own genetic material, even when egg quality has significantly declined due to age. Unlike egg donation, which results in a genetically unrelated child, MRT retains maternal nuclear DNA—meaning your child carries your genetic heritage.
What if MRT Doesn’t Work? Can I Go Through a Tandem IVF Cycle?
Yes, absolutely—you can go through a tandem IVF cycle alongside your MRT treatment, and in fact, this is something we actively recommend in certain clinical situations.
In mitochondrial replacement therapy (MRT), only the cytoplasmic component (mitochondria) from the donor egg is used, while your own nuclear genetic material is retained. Because the resulting child will be genetically yours, phenotypic matching with the donor is not necessary in MRT cycles. The donor’s mitochondria are purely functional and do not contribute to physical traits or personality characteristics.
However, in cases where we anticipate a lower probability of success—for example, due to very low egg yield or severely diminished oocyte quality—a tandem IVF cycle can be considered as a backup plan. This means we perform a simultaneous IVF cycle using donor oocytes alongside your MRT cycle. This offers the advantage of creating two separate groups of embryos:
• One group derived from your own genetic material via MRT
• Another group derived from donor eggs.
How Tandem IVF Works Alongside MRT?
• Ovarian stimulation and retrieval are carried out for you to obtain oocytes for MRT.
• In parallel, we coordinate with a suitable donor for a fresh egg donation cycle.
• Your oocytes undergo maternal spindle transfer (MST) and are fertilized via ICSI.
• The donor oocytes are fertilized with your partner’s (or selected donor’s) sperm to create a second set of embryos.
• All embryos are cultured to the blastocyst stage, at which point:
• One or more embryos—either from MRT or donor IVF—can be selected for immediate transfer.
• Remaining embryos from either group can be vitrified (frozen) and stored for future use.
A major benefit of the tandem approach is that you retain full flexibility. Depending on embryo quality and your treatment preferences, you may:
• Choose to transfer only your own (MRT-derived) embryo
• Choose to transfer a donor egg embryo
• Or opt for dual embryo transfer—one from each group, though this carries a higher chance of twins.
Any remaining embryos can be safely frozen for future cycles, giving you options down the road without the need to repeat stimulation or donor matching.
Tandem IVF provides:
• Reassurance and backup in case MRT embryos do not develop optimally
• Higher cumulative pregnancy rates by increasing the pool of viable embryos or just the peace of mind of having embryos available for transfer should own eggs do not work.
• Time efficiency, since both strategies are executed in a single treatment window
• Financial efficiency since you wouldn’t need to go through an additional separate IVF cycle using donor oocytes, which would impose a higher cost.
Ready to Learn More?
If you’ve been told your chances of success with your own eggs are too low, or if you’ve experienced repeated IVF failures, MRT may offer a renewed path to parenthood. Contact us to schedule a consultation or to begin your evaluation.
مركز شمال قبرص لأطفال الأنابيب
مركز شمال قبرص لأطفال الأنابيب هو عيادة خصوبة تركز على المريض ، ويقع ضمن “مستشفى النخبة للأبحاث والجراحة"في نيقوسيا، قبرص. تعد عيادتنا واحدة من عيادات الخصوبة الأكثر تقدمًا في العالم، حيث تقدم مجموعة واسعة من خيارات العلاج بأسعار معقولة.
اتصل-بنا
هاتف
إنجليزي: +90 548 875 8000
فرنسي: +90 548 876 8000
اللغة التركية: +90 542 869 8000
عربي: +90 548 875 8000
ألمانية: +90 548 830 1987
الروسية: +90 548 828 9955
بريد إلكتروني
info@northcyprusivf.net
© 2020 LowCostIVF - جميع الحقوق محفوظة. سياسة الخصوصية